TNFRSF9
-
Official Full Name
tumor necrosis factor receptor superfamily, member 9 -
Overview
Human 4-1BB Ligand Receptor, also known as the Tumor Necrosis Factor Receptor Superfamily Member 9, is a 255 amino acid cytokine protein implicated in T-cell activation pathways. After initial synthesis of the peptide, the cytokine is proteolytically cleaved by proteases, thus separating the 23 residue signal sequence from the actual mature 232 residue 4-1BB Receptor sequence. The protein is expressed from the TNFRSF9 gene located at locus 1p36 on chromosome 1. The 4-1BB receptor or TNFRSF9 inherently has high affinity binding for the 4-1BB ligand such that upon binding, the receptor contributes to clonal expansion, survival and development of T-cells. It can also induce proliferation in peripheral monocytes, enhance T-cell apoptosis induced by TCR/CD3 triggered activation and regulate CD28 co-stimulation to promote Th1 cell responses. The expression of this receptor is induced by lymphocyte activation. TRAF adaptor proteins such as TRAF1, TRAF2 and TRAF 3 have been shown to bind to this receptor and transducer the signals leading to activation of NF-κB. -
Synonyms
TNFRSF9;4-1BB;CD137;CDw137;ILA;CD137 antigen;T cell antigen ILA;T-cell antigen 4-1BB homolog;T-cell antigen ILA;homolog of mouse 4-1BB;induced by lymphocyte activation (ILA);interleukin-activated receptor, homolog of mouse Ly63;receptor protein 4-1BB;tumor necrosis factor receptor superfamily member 9
Recombinant Proteins
- Human
- Monkey
- Canine
- Mouse
- Dog
- Rabbit
- Rhesus macaque
- Rat
- Cynomolgus/Rhesus macaque
- Cynomolgus
- HEK293
- Human Cells
- CHO
- Mammalian Cells
- E.coli
- Insect Cells
- NS0
- Fc
- His
- Avi
- rFc
- Non
- hIgG4
- Flag
- GST
- mIgG2a
Background
What is TNFRSF9 protein?
TNFRSF9 (TNF receptor superfamily member 9) gene is a protein coding gene which situated on the short arm of chromosome 1 at locus 1p36. Human 4-1BB Ligand Receptor, also known as the Tumor Necrosis Factor Receptor Superfamily Member 9, is a 255 amino acid cytokine protein implicated in T-cell activation pathways. After initial synthesis of the peptide, the cytokine is proteolytically cleaved by proteases, thus separating the 23 residue signal sequence from the actual mature 232 residue 4-1BB Receptor sequence. The TNFRSF9 protein is consisted of 255 amino acids and its molecular mass is approximately 27.9 kDa.
What is the function of TNFRSF9 protein?
The 4-1BB receptor or TNFRSF9 inherently has high affinity binding for the 4-1BB ligand such that upon binding, the receptor contributes to clonal expansion, survival and development of T-cells. It can also induce proliferation in peripheral monocytes, enhance T-cell apoptosis induced by TCR/CD3 triggered activation and regulate CD28 co-stimulation to promote Th1 cell responses. The expression of this receptor is induced by lymphocyte activation. TRAF adaptor proteins such as TRAF1, TRAF2 and TRAF 3 have been shown to bind to this receptor and transducer the signals leading to activation of NF-κB.
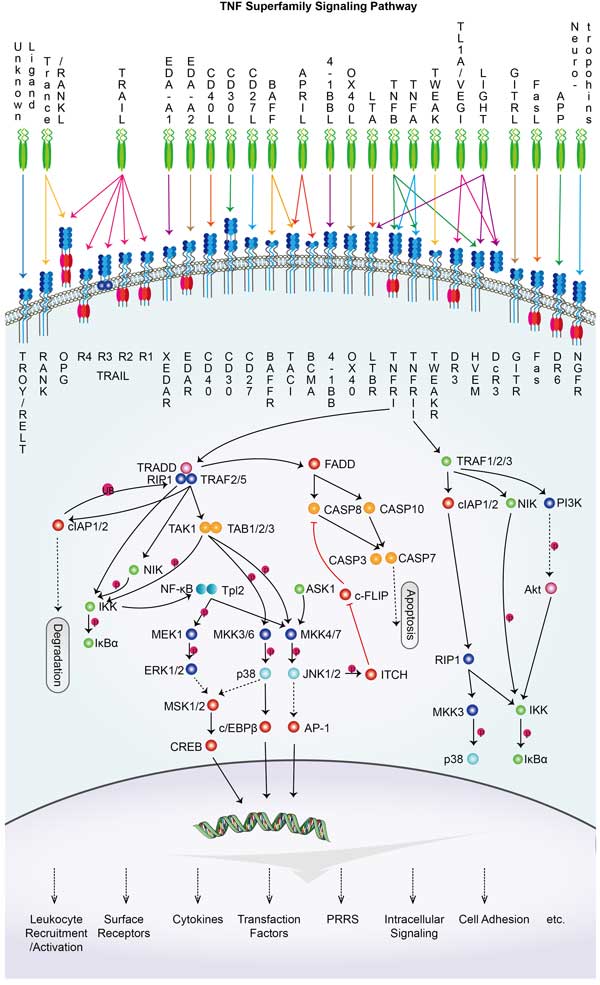
TNFRSF9 Related Signaling Pathway
TNFRSF9 (also known as CD137 or 4-1BB) protein is a receptor protein on the cell membrane, mainly through the binding of its ligand CD137L to activate downstream signaling pathways, involved in a variety of immune responses and cell-mediated immune regulation processes. Signaling pathways involved in TNFRSF9 protein mainly include: NF-κB signaling pathway: Activation of TNFRSF9 protein can trigger activation of the NF-κB signaling pathway, leading to a series of NF-κB-mediated inflammatory responses and cell proliferation processes in the cell.
PI3K/AKT signaling pathway: TNFRSF9 protein can also activate the PI3K/AKT signaling pathway to promote cell survival, proliferation and metabolism.
MAPK/ERK signaling pathway: Activation of TNFRSF9 protein can also activate the MAPK/ERK signaling pathway, affecting cell proliferation and survival.
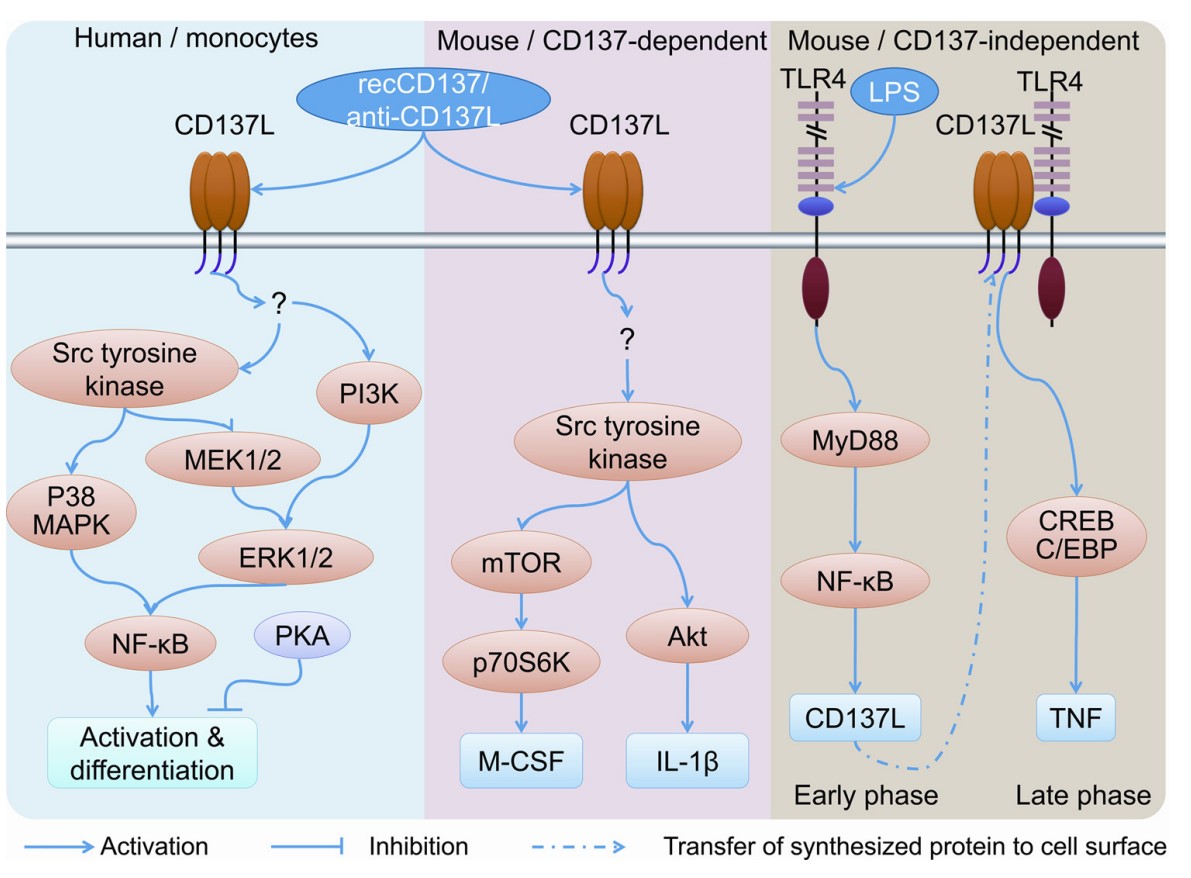
Fig1. CD137 ligand signaling pathways in man and mouse. (Zhe Shao, 2011)
TNFRSF9 Related Diseases
TNFRSF9 plays an important role in immune system function. Therefore, diseases related to CD137 protein mainly include: immune diseases such as rheumatoid arthritis, systemic lupus erythematosus and so on. The CD137 protein plays a role in regulating the function of T and NK cells and is therefore relevant for tumor immunotherapy. CD137 signaling pathway is involved in regulating the body's immune response to infection and is associated with viral and bacterial infections. It is also associated with a number of other diseases, such as multiple sclerosis, inflammatory bowel disease, etc. These diseases are usually caused by an abnormal response of the immune system.
Bioapplications of TNFRSF9
TNFRSF9 protein has a wide range of applications in immunotherapy, cancer therapy and the treatment of autoimmune diseases. Several drug and immune cell engineering techniques have been used for cancer treatment research and development using TNFRSF9 proteins.
Case Study
Case study 1: Kang Yi Lee, 2021
Rhabdomyosarcoma (RMS) is a heterogeneous soft tissue neoplasm most frequently found in children and adolescents. As the prognosis for recurrent and metastatic RMS remains poor, immunotherapies are hoped to improve quality of life and survival. But it was puzzling to find expression of CD137 on an RMS tissue microarray by multiplex staining. Functional in vitro experiments demonstrate that CD137 on RMS cells is being transferred to adjacent antigen-presenting cells by trogocytosis, where it downregulates CD137 ligand, and thereby reduces T cell costimulation which results in reduced killing of RMS cells. The transfer of CD137 and the subsequent downregulation of CD137 ligand is a physiological negative feedback mechanism that is likely usurped by RMS, and may facilitate its escape from immune surveillance. This study implicates ectopic CD137 expression as a pathogenesis mechanism in RMS, and it demonstrates that CD137 may be a novel target for immunotherapy of RMS.
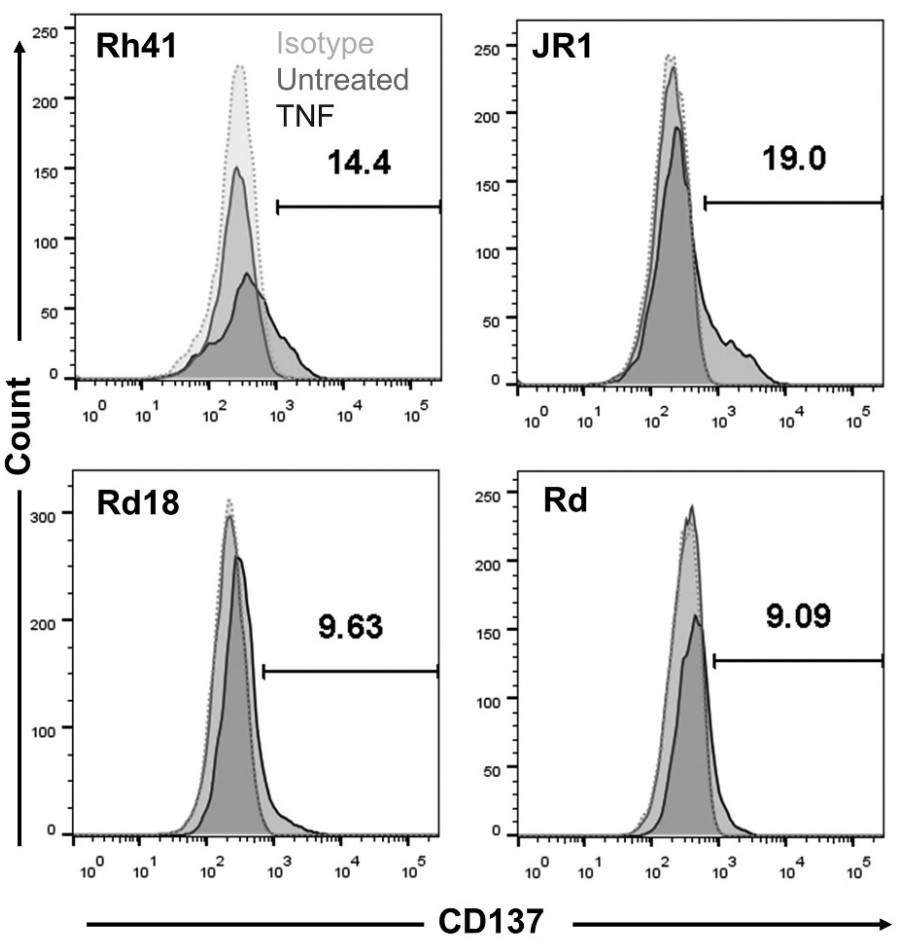
Fig1. Rd18, Rh41, Rd and JR1 cells were cultured with 75 ng/ml of TNF for 24 h. CD137 expression was measured by flow cytometry.
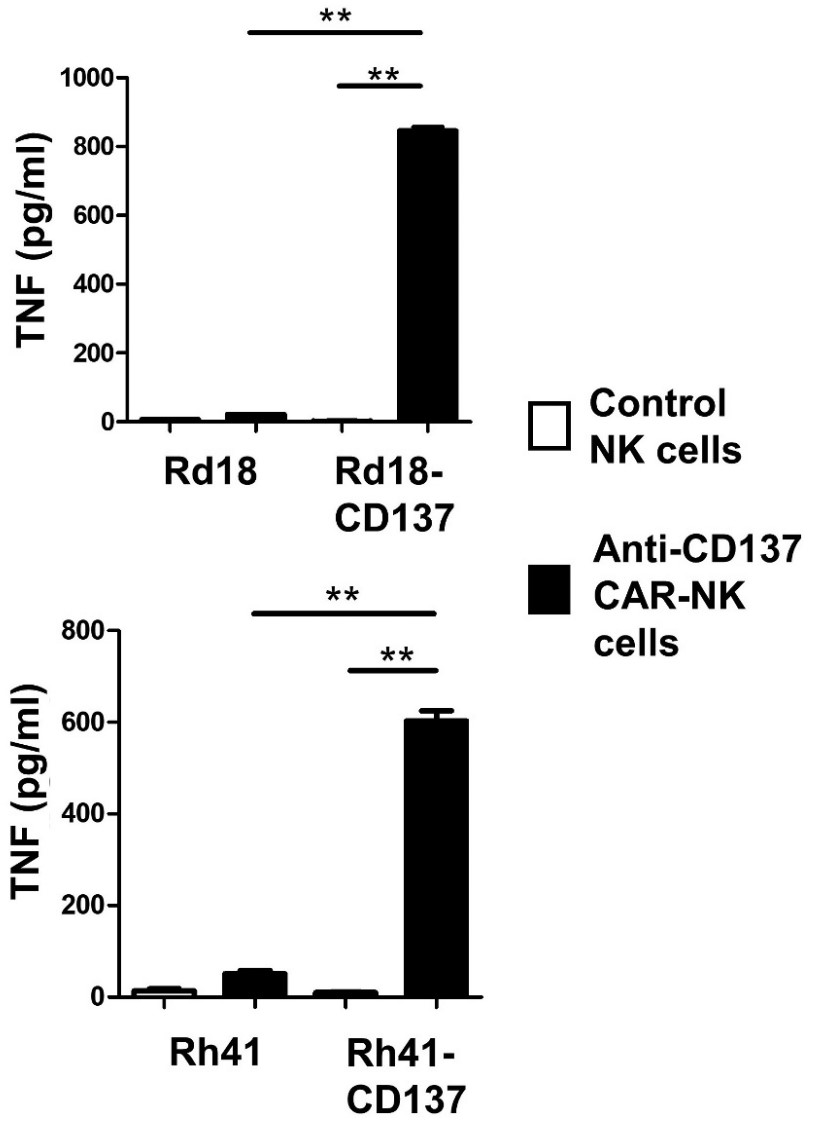
Case study 2: Benjamin I Philipson, 2020
Clinical response to chimeric antigen receptor (CAR) T cell therapy is correlated with CAR T cell persistence, especially for CAR T cells that target CD19+ hematologic malignancies. 4-1BB-costimulated CAR (BBζ) T cells exhibit longer persistence after adoptive transfer than do CD28-costimulated CAR (28ζ) T cells. 4-1BB signaling improves T cell persistence even in the context of 28ζ CAR activation, which indicates distinct prosurvival signals mediated by the 4-1BB cytoplasmic domain. To specifically study signal transduction by CARs, we developed a cell-free, ligand-based activation and ex vivo culture system for CD19-specific CAR T cells.
This study showed that only BBζ CARs activated noncanonical nuclear factor κB (ncNF-κB) signaling in T cells basally and that the anti-CD19 BBζ CAR further enhanced ncNF-κB signaling after ligand engagement. Although the findings do not exclude the importance of other signaling differences between BBζ and 28ζ CARs, they demonstrate the necessary and nonredundant role of ncNF-κB signaling in promoting the survival of BBζ CAR T cells.
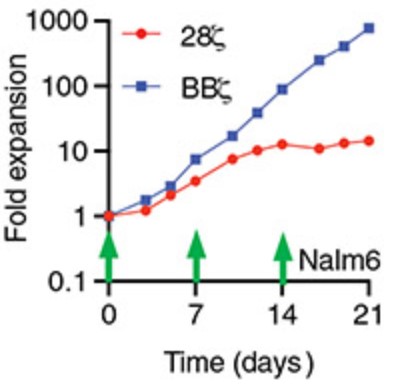
Fig3. Representative ex vivo expansion of the indicated CAR T cells stimulated by irradiated Nalm6 target cells at the indicated times at a T cell to target cell ratio of 2:1.
Quality Guarantee
High Purity
Fig1. SDS-PAGE (TNFRSF9-517H) (PROTOCOL for western blot)
.
Fig2. SDS-PAGE (TNFRSF9-345H) (PROTOCOL for western blot)
Involved Pathway
TNFRSF9 involved in several pathways and played different roles in them. We selected most pathways TNFRSF9 participated on our site, such as Cytokine-cytokine receptor interaction, which may be useful for your reference. Also, other proteins which involved in the same pathway with TNFRSF9 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Cytokine-cytokine receptor interaction | IL17RA,LTA,CTF1,CXCL1,Ifna11,GM1987,TSLP,IL8L2,TGFBR1,Ifnlr1 |
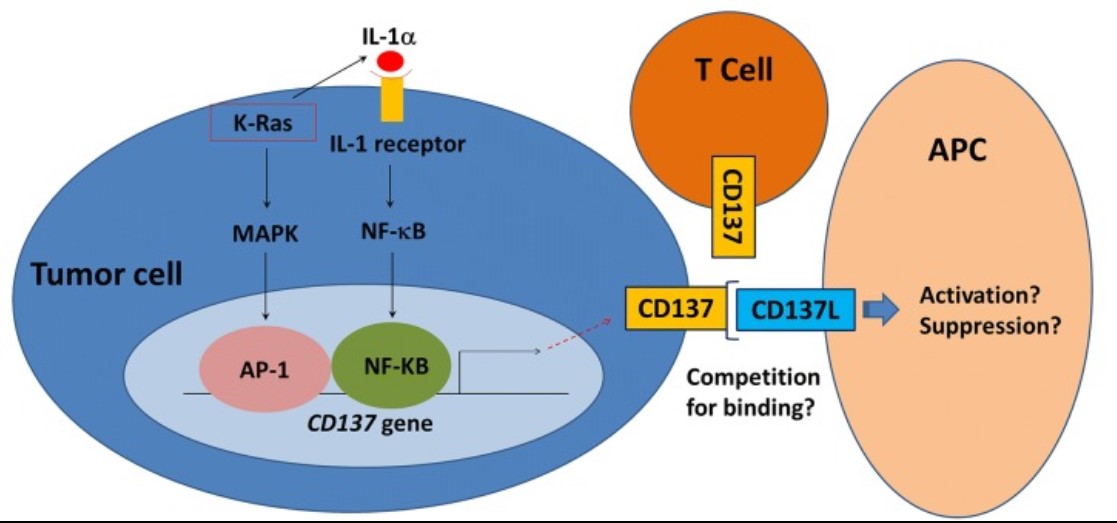
Fig1. Schematic model depicting the likely molecular mechanisms by which K-Ras regulates CD137 gene transcription. (Christophe Glorieux, 2019)
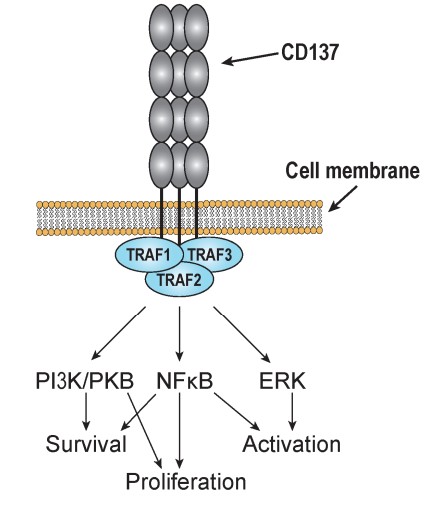
Fig2. Signal transduction of CD137 in T cells. Ligation of the trimerized receptor promotes recruitment of tumor necrosis factor receptor associated factors (TRAFs). (Leif Å Söderström, 2018)
Protein Function
TNFRSF9 has several biochemical functions, for example, receptor activity,tumor necrosis factor-activated receptor activity. Some of the functions are cooperated with other proteins, some of the functions could acted by TNFRSF9 itself. We selected most functions TNFRSF9 had, and list some proteins which have the same functions with TNFRSF9. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| receptor activity | GRIK5,PROCR,ATP6AP2,LRP2,NEO1,RET,IL10RA,GUCY1A3,LDLR,ANXA2R |
| tumor necrosis factor-activated receptor activity | NRADD,TNFRSF25,LTBR,TNFRSF14,TNFRSF10D,TNFRSF1B,TNFRSF19,TNFRSFA,TNFRSF10B,TNFRSF11B |
Interacting Protein
TNFRSF9 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with TNFRSF9 here. Most of them are supplied by our site. Hope this information will be useful for your research of TNFRSF9.
TNFRSF9 Related Signal Pathway
Resources
Research Area
Related Services
Related Products
References
- Campana, D; Schwarz, H; et al. 4-1BB Chimeric Antigen Receptors. CANCER JOURNAL 20:134-140(2014).
- Song, C; Sadashivaiah, K; et al. Eomesodermin is required for antitumor immunity mediated by 4-1BB-agonist immunotherapy. ONCOIMMUNOLOGY 3:-(2014).