B Cell Chemotaxis, Migration, and Adhesion
Related Symbol Search List
- CD48
- CD147
- CCL19
- CCL21
- CCRL2
- CXCL12
- CXCL13
- CXCR3
- CXCR4
- CXCR5
- IGSF8
- ITGA4
- ITGAM
- ITGAX
- LTBR
- L1CAM
- LTB4R
- LTB4R2
- S1PR1
- L-selectin
- Selplg
Immunology Background
Background
B-cell chemotaxis, migration, and adhesion are essential processes that allow B cells to navigate through tissues, interact with antigen-presenting cells (APCs), and ultimately participate in immune responses. Here's an overview of these processes:
Chemotaxis
B-cell chemotaxis refers to the directed movement of B cells in response to chemical gradients. Chemokines, small signaling proteins, play a crucial role in guiding B-cell migration. Chemokines are secreted by various cells, including APCs, and bind to specific chemokine receptors on B cells. This interaction triggers intracellular signaling pathways that lead to changes in cytoskeletal dynamics, allowing B cells to migrate towards higher concentrations of chemokines. Chemokine receptors commonly expressed on B cells include CXCR4, CXCR5, and CCR7.
Migration
B-cell migration involves the movement of B cells from one location to another, such as from the bloodstream to lymphoid tissues or from follicles to germinal centers. B cells can migrate through different mechanisms:
- Transendothelial migration: B cells can cross the endothelial barrier to exit blood vessels and enter tissues. This process involves interactions between adhesion molecules, such as selectins and integrins, expressed on B cells and endothelial cells, as well as chemokine gradients.
- Lymphocyte homing: B cells express specific adhesion molecules, such as L-selectin and integrins (e.g., LFA-1), which allow them to adhere to and migrate across high endothelial venules (HEVs) in lymphoid tissues. This process is critical for B-cell entry into lymph nodes and other lymphoid organs.
- Chemotactic migration: As mentioned earlier, B cells can migrate in response to chemokine gradients. For example, CXCR5 expressed on B cells guides their migration towards follicles in lymphoid tissues, where the chemokine CXCL13 is abundant.
Adhesion
Adhesion molecules play a vital role in facilitating interactions between B cells and other cells, including APCs, during immune responses. These interactions are essential for antigen capture, presentation, and activation of B cells. Some key adhesion molecules involved in B-cell adhesion are integrins, selectins, immunoglobulin superfamily (IgSF) molecules.
Overall, B-cell chemotaxis, migration, and adhesion are finely regulated processes that allow B cells to navigate through tissues, respond to chemotactic cues, establish interactions with APCs, and contribute to immune responses. These processes are critical for B-cell activation, antibody production, and the generation of immune memory.
Molecules Associated with B Cell Chemotaxis, Migration, and Adhesion
Several molecules are associated with B-cell chemotaxis, migration, and adhesion. These molecules play crucial roles in guiding B-cell movement, facilitating interactions with other cells, and promoting adhesion to various surfaces. Here are some key molecules involved in these processes:
| Types | Functions |
|---|---|
| Chemokines | Chemokines are small signaling proteins that act as chemoattractants for B cells. They bind to specific chemokine receptors on B cells, initiating intracellular signaling pathways that direct cell movement. Examples include CXCL12 (binds to CXCR4), CXCL13 (binds to CXCR5), and CCL19/CCL21 (bind to CCR7). |
| Chemokine Receptors | B cells express chemokine receptors, which enable them to respond to chemotactic gradients. Examples of chemokine receptors on B cells include CXCR3, CXCR4, CXCR5, CCR7, LTB4R, LTB4R2, and S1PR1. |
| Cell Adhesion Related Molecules | Cell adhesion related molecules, such as integrins, selectins, and cadherins, help B cells interact with other cells and the extracellular matrix during the processes of chemotaxis, migration, and adhesion. Including L-selectin (CD62L), LFA-1 (αLβ2, CD11a/CD18), VLA-4 (α4β1, CD49d/CD29), CD48, CD147, CCRL2, IGSF8, ITGA4, ITGAM, ITGAX, L-selectin, Selplg, L1CAM, LTBR. |
These molecules, along with their receptors and ligands, orchestrate the intricate processes of B-cell chemotaxis, migration, and adhesion. They enable B cells to navigate through tissues, respond to chemotactic cues, establish interactions with other immune cells, and contribute to immune responses.
Techniques for Studying B Cell Chemotaxis, Migration, and Adhesion
Studying B cell chemotaxis, migration, and adhesion involves a combination of experimental techniques and assays that allow researchers to investigate these processes in a controlled laboratory setting. Here is an overview of some commonly used techniques:
| Techniques | Details |
|---|---|
| Transwell Migration Assay | The transwell migration assay is a widely used technique to study B cell chemotaxis. It involves the use of a porous membrane with defined pore sizes that separates two chambers. B cells are applied to the upper chamber, and a chemoattractant, such as a chemokine, is added to the lower chamber. B cells migrate through the membrane towards the chemoattractant, and their migration can be quantified by counting the cells that reach the lower chamber. This assay provides information about the chemotactic response of B cells to specific stimuli. |
| Boyden Chamber Assay | The Boyden chamber assay is similar to the transwell migration assay and is used to study B cell migration. It consists of two chambers separated by a porous membrane. B cells are placed in the upper chamber, and the lower chamber contains a gradient of chemoattractant. B cells migrate through the membrane, and the number of cells that reach the lower chamber is quantified. The Boyden chamber assay allows for the assessment of B cell migration in response to gradients of chemotactic stimuli. |
| Immunofluorescence and Confocal Microscopy | Immunofluorescence microscopy combined with confocal microscopy enables the visualization and characterization of B cell migration and adhesion processes in tissues. B cells can be labeled with fluorescent dyes or antibodies against specific markers and then imaged in tissues or in vitro setups. This technique provides insights into the localization, movement, interactions, and adhesion of B cells within complex tissue environments. |
| Flow Cytometry | Flow cytometry is a versatile technique that can be used to study B cell migration and adhesion. It allows for the analysis of surface markers and adhesion molecules on B cells, providing information about their adhesive properties and activation states. Flow cytometry can also be used to measure the expression levels of chemokine receptors on B cells, enabling the assessment of their potential responsiveness to chemotactic signals. |
| Adhesion Assays | Various adhesion assays can be employed to study B cell adhesion to other cells or extracellular matrix components. These assays include static adhesion assays, where B cells are allowed to adhere to a substrate in a controlled environment, and flow-based adhesion assays, where B cells are flowed over a substrate under defined shear stress conditions. These assays measure the extent of adhesion and can be combined with imaging or flow cytometry to characterize the molecules involved in B cell adhesion. |
| Live Cell Imaging | Live cell imaging techniques, such as time-lapse microscopy and video microscopy, enable the real-time visualization of B cell migration, chemotaxis, and adhesion. These techniques provide dynamic information about cell movement, interactions, and changes in morphology over time. Live cell imaging can be combined with fluorescent labeling or genetic reporters to track specific molecules or signaling events involved in B cell migration and adhesion. |
| Genetic and Pharmacological Approaches | Genetic manipulation of B cells, such as the knockout or overexpression of specific genes, can provide insights into the molecular mechanisms underlying B cell chemotaxis, migration, and adhesion. Additionally, pharmacological inhibitors or activators can be used to modulate signaling pathways or adhesion molecule functions to study their roles in B cell migration and adhesion. |
The combination of these techniques allows researchers to investigate the complex processes of B cell chemotaxis, migration, and adhesion. By understanding these processes, researchers can gain insights into the regulation and functional consequences of B cell movement, which is crucial for immune responses, antibody production, and the development of targeted therapeutics.
Targeted Therapy and Therapeutic Targets for B-Cell Chemotaxis, Migration, and Adhesion
Manipulating B cell migration is a promising strategy for the treatment of autoimmune diseases, cancer, and infectious diseases. Understanding the mechanisms underlying B cell migration is crucial for the development of novel therapeutic approaches. Here's a discussion of potential strategies and the importance of targeting B cell migration in these disease contexts:
 Fig.2 Imaging and analysis of single cell migration. (Le Dévédec SE, et al., 2010)
Fig.2 Imaging and analysis of single cell migration. (Le Dévédec SE, et al., 2010)Autoimmune Diseases
Autoimmune diseases involve aberrant immune responses where B cells play a significant role. In diseases such as rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus, B cells migrate to target tissues, contribute to inflammation, and produce autoantibodies. Targeting B cell migration can help limit their infiltration into affected tissues, reducing local inflammation and tissue damage.
In this context, strategies include targeting chemokine receptors, such as CXCR5 and CCR7, to interfere with the migration of autoreactive B cells to inflammatory sites.
Cancer
B cells can have both anti-tumor and pro-tumor functions in cancer. Understanding the migratory behavior of B cells within the tumor microenvironment is critical for developing effective immunotherapies. In some cancers, B cells create an immunosuppressive environment or promote tumor growth and metastasis. Targeting B cell migration can hinder their interaction with tumor cells and inhibit tumor-promoting functions.
Strategies may involve blocking integrins, chemokine receptors, or adhesion molecules involved in B cell trafficking to the tumor site.
Infectious Diseases
B cells play a crucial role in the immune response against infectious pathogens. However, in certain infections, dysregulated B cell migration can contribute to disease pathogenesis. For example, in chronic viral infections like HIV or hepatitis C, B cells can accumulate in lymphoid tissues and fail to migrate to sites of infection. Modulating B cell migration in infectious diseases can help enhance the immune response and improve pathogen clearance.
Targeting chemokine receptors or adhesion molecules involved in B cell migration can potentially enhance B cell trafficking to infected tissues.
The importance of understanding B cell migration in the development of novel therapies cannot be overstated. By studying the migratory behavior of B cells, researchers can identify specific molecules, receptors, and signaling pathways that can be targeted for therapeutic intervention. Such targeted therapies can modulate B cell trafficking, prevent their infiltration into disease-affected tissues, disrupt interactions with other cells, and regulate immune responses.
Additionally, understanding B cell migration can aid in the design and optimization of immunotherapies, such as chimeric antigen receptor (CAR) T-cell therapy or antibody-based therapies, by considering the migration patterns and homing capabilities of B cells. This knowledge can help guide the engineering of therapeutic agents to enhance their efficacy in targeting B cells in specific disease contexts.
Case Study
Case 1: Guo J, Xu Z, Gunderson RC, Xu B, Michie SA. LFA-1/ICAM-1 Adhesion Pathway Mediates the Homeostatic Migration of Lymphocytes from Peripheral Tissues into Lymph Nodes through Lymphatic Vessels. Biomolecules. 2023;13(8):1194.
To investigate the role of ICAM-1 in lymphocyte migration from peripheral tissues into draining LNs, the authors transferred WT donor lymphocytes into the peripheral tissues of WT and ICAM-1-/- mice. WT donor T and B cells migrated from the skin and lungs into the draining LNs in ICAM-1-/- mice < 30% as efficiently as in WT mice (A, B, D). Similarly, WT T and B cells migrated from the peritoneum into the PanLN of ICAM-1-/- mice at approximately 30% and 50%, respectively, as efficiently as in WT mice (A, B, D). In contrast, donor T cells migrated from blood vessel HEVs into LNs in ICAM-1-/- host mice equally well, or even slightly better than they did in WT host mice 2 h after i.v. transfer (C). There was a slight reduction in the migration of donor B cells from blood vessel HEVs into CLN, but not the BLN and PanLN of ICAM-1-/- host mice as compared to WT host mice (E). These results indicate that ICAM-1 is important for the migration of lymphocytes from peripheral tissues into LNs, but not for the migration of most lymphocytes from blood vessels into LNs.
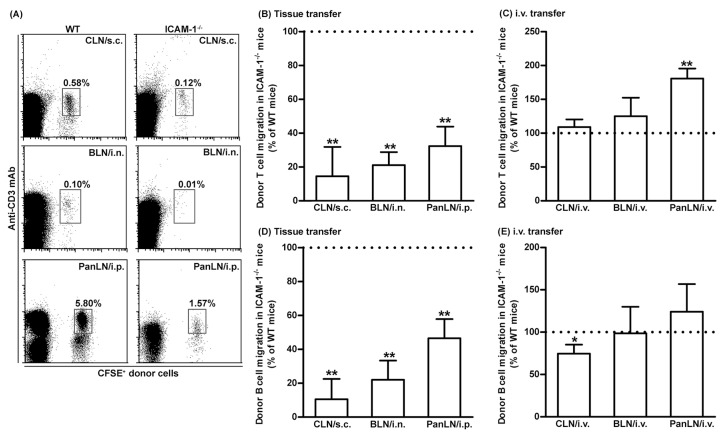 Fig.1 Intercellular adhesion molecules-1 (ICAM-1) is important for the migration of T and B cells from peripheral tissues into draining lymph nodes (LNs).
Fig.1 Intercellular adhesion molecules-1 (ICAM-1) is important for the migration of T and B cells from peripheral tissues into draining lymph nodes (LNs).Case 2: Nunes J, Tafesse R, Mao C, et al. Siglec-6 as a therapeutic target for cell migration and adhesion in chronic lymphocytic leukemia. Nat Commun. 2024;15(1):5180.
CLL-specific overexpression of Siglec-6 led authors to hypothesize a potential role for Siglec-6 in the migration of B-CLL cells to the tumor microenvironmental (TME) niche. The JML-1 Ab blocked attachment of Siglec-6+ primary B-CLL cells to sTn+ CLL-BMSCs in an in vitro co-culture assay(a), indicating the need for Siglec-6 in attachment of B-CLL cells to CLL-BMSCs. Importantly, the JML-1 Ab had no effect on attachment of Siglec-6− normal donor B cells to CLL-BMSCs (b). The JML-1 Ab also had no effect on attachment of B-CLL cells to normal BMSCs which lack Siglec-6 ligand sTn (c). Further, CLL-BMSCs promoted in vitro migration of B-CLL cells in a transwell migration assay, which was in turn inhibited by the JML-1 Ab (d). Thus, JML-1 Ab could block both in vitro migration as well as adhesion of B-CLL cells to CLL-BMSCs. The JML-1 Ab could also negate in vitro migration of Siglec-6+ MEC1-002 cells. A comparison between in vitro migration of MEC1-002 WT cells and primary CLL cells with or without Siglec-6 blockade with JML-1 Ab. To test if biological inhibition of Siglec-6 is sufficient to inhibit migration of MEC1-002 cells, the authors used the CRISPR-Cas9 technique to establish stable knockout of SIGLEC-6 in MEC1-002 cells (b). Consistent with the observations in primary CLL cells, knocking out SIGLEC-6 in MEC1-002 cells resulted in significant reduction in migration towards sTn+ CLL-BMSCs (e). These results thus suggest a migratory and adhesion role of Siglec-6 in B-CLL cells.
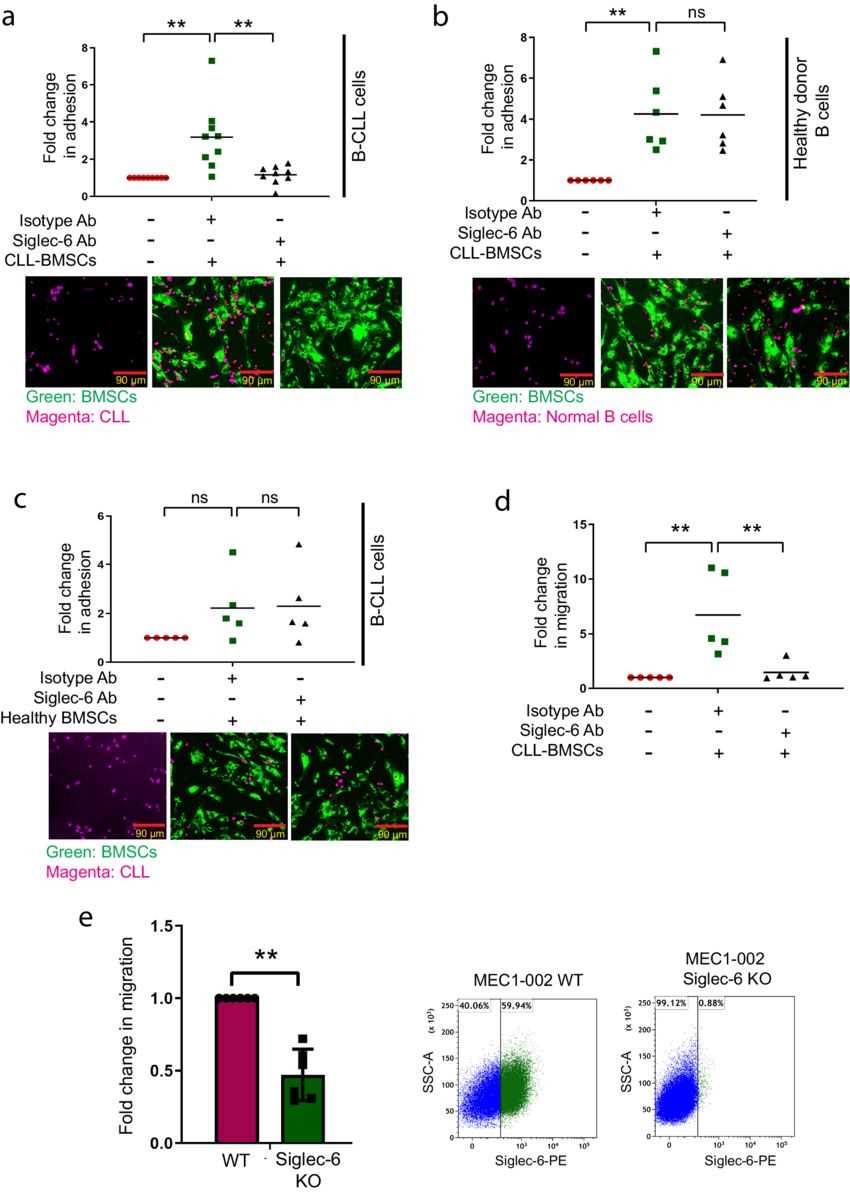 Fig.2 Siglec-6 promotes migration and adhesion of chronic lymphocytic leukemia (CLL) cells in vitro.
Fig.2 Siglec-6 promotes migration and adhesion of chronic lymphocytic leukemia (CLL) cells in vitro.References
- Conway JRW, Jacquemet G. Cell matrix adhesion in cell migration. Essays Biochem. 2019;63(5):535-551.
- Le Dévédec SE, Yan K, de Bont H, et al. Systems microscopy approaches to understand cancer cell migration and metastasis. Cell Mol Life Sci. 2010;67(19):3219-3240.
- Severinson E, Westerberg L. Regulation of adhesion and motility in B lymphocytes. Scand J Immunol. 2003;58(2):139-144.

