Cas9 Protein: Unveiling the "Molecular Scissors" of Gene Editing
Introduction
In the field of life sciences, gene editing technology resembles a magical key that opens the door for humans to deeply explore and modify biological genetic information. It not only assists scientists in uncovering the mysteries of genes in fundamental research but also shows immense potential for applications in medicine, agriculture, biotechnology, and various other fields. By precisely modifying the genes of organisms, we aim to cure genetic diseases that have troubled humanity for ages, cultivate crops with desirable traits, and even provide new strategies for conserving endangered species.
Among the many tools for gene editing, the Cas9 protein undeniably stands out as one of the most brilliant stars and is hailed as the "molecular scissors" of gene editing. It holds a pivotal position in gene editing technology. The advent of the Cas9 protein has made gene editing more efficient, precise, and user-friendly, advancing the transition of gene editing technology from the laboratory to practical applications and becoming an indispensable tool in contemporary life science research.
The Discovery of Cas9 Protein
The discovery of the Cas9 protein originated from in-depth studies of the bacterial immune system. Over a lengthy evolutionary process, bacteria gradually developed a unique defense mechanism, known as the CRISPR-Cas system, to resist invasions from bacteriophages and other viruses. As early as the 1980s, scientists discovered a particular repeating sequence, then termed clustered regularly interspaced short palindromic repeats (CRISPR), in the genomes of Escherichia coli, although its function was not yet understood.
It wasn't until the early 21st century, with the continuous advancement in microbial genome research, that scientists gradually unveiled the mysteries of the CRISPR-Cas system. In 2002, researchers found that CRISPR, along with a series of associated proteins (Cas proteins), formed the adaptive immune system of bacteria. When a bacteriophage infects a bacterium, the bacterium integrates fragments of the phage DNA into its CRISPR sequences, forming a memory. Upon re-encountering the same phage, CRISPR transcribes RNA, which, in conjunction with Cas proteins, can recognize and cleave the phage DNA, thereby preventing infection.
Among the numerous Cas proteins, Cas9 garners extensive attention due to its unique functionality and user-friendly characteristics. In 2012, scientists including Jennifer Doudna and Emmanuelle Charpentier, through thorough research on the CRISPR-Cas system in Streptococcus, elucidated the mechanism of Cas9 protein function. They discovered that guided by a small RNA (guide RNA, sgRNA), the Cas9 protein can precisely recognize and cleave specific DNA sequences. This discovery not only unveiled the operational principles of the bacterial immune system but also brought about a revolutionary breakthrough in gene editing technology, heralding the era of CRISPR-Cas9 gene editing.
Detailed Analysis of Cas9 Protein Structure and Function Domains
Cas9 protein is a relatively large protein molecule with a complex structure and high specificity, composed of multiple functional domains that collaborate to accomplish gene editing tasks.
DNA Recognition Domains
The Cas9 protein contains two main DNA recognition domains: the REC domain (Repeat-binding domain) and the Bridge Helix domain. The REC domain binds to the guide RNA and undergoes a conformational change upon binding to recognize the target DNA sequence. A specific sequence on the guide RNA (about 20 nucleotides) complements the target DNA sequence through base pairing, with the REC domain facilitating accurate localization to the target DNA region through its interaction with the guide RNA.
The Bridge Helix domain plays a crucial role as a bridge in the binding process between the Cas9 protein and DNA. It senses structural changes in the DNA double helix, ensuring correct binding between the Cas9 protein and DNA, and stabilizing the complex structure during recognition to prepare for the subsequent cleavage reaction.
DNA Cleavage Active Sites
The Cas9 protein has two active sites for DNA cleavage: the HNH nuclease domain and the RuvC nuclease domain. The HNH domain cleaves the DNA strand (target strand) complementary to the guide RNA, while the RuvC domain cleaves the non-complementary strand (non-target strand). These two active sites, akin to two sharp "scissors," collaborate to cleave the double-stranded DNA, forming double-strand breaks (DSB) upon recognizing the target DNA sequence.
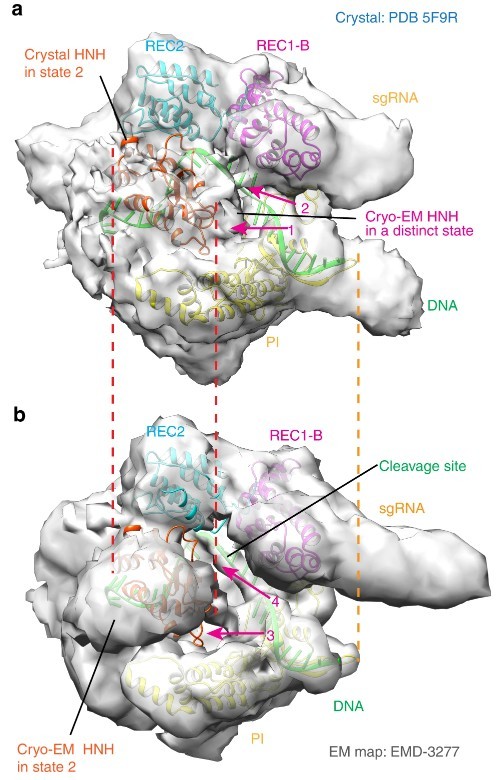 Fig2. The conformational state 3 of the HNH domain revealed by the cryo-EM
Fig2. The conformational state 3 of the HNH domain revealed by the cryo-EMOther Functional Domains
Besides the primary functional domains mentioned above, the Cas9 protein includes other auxiliary functional domains, such as the PAM recognition domain. The PAM (Protospacer Adjacent Motif) is a specific short sequence adjacent to the target DNA sequence (usually NGG, where N is any nucleotide) that is crucial for the Cas9 protein's recognition of the target DNA sequence. The PAM recognition domain can specifically identify the PAM sequence. Only when a correct PAM sequence is present downstream of the target DNA can the Cas9 protein efficiently bind and cleave the DNA. This property ensures Cas9 protein specificity during gene editing, preventing erroneous cleavage of non-target sequences.
Featured Cas9 Protein
| Cat.No. | Product Name | Source | Species | Tag |
|---|---|---|---|---|
| CAS9-22S | Active Recombinant Full Length Streptococcus pyogenes serotype M1 type II CRISPR RNA-guided endonuclease Cas9 Protein, GFP-tagged | E.coli | Streptococcus pyogenes serotype M1 | GFP |
| Cas9 -121S | Recombinant CRISPR Cas9 protein | E.coli | Non | |
| cas9-12S | Active Recombinant Streptococcus pyogenes M1 cas9 Protein, His-tagged | Insect Cells | Streptococcus pyogenes M1 | His |
| cas9-11S | Recombinant Streptococcus pyogenes cas9 protein | E.coli | Streptococcus pyogenes | Non |
| cas9-12 | Recombinant Cas9 Nuclease Protein | E.coli | Non | |
| cas9-85H | Recombinant bacterial Cas9 protein, His-tagged | E.coli | Streptococcus pyogenes serotype M1 | His |
| cas9-01S | Recombinant S. pyogenes Cas9 Protein, GFP-Labeled | E.coli | Streptococcus pyogenes |
Mechanism of Cas9 Protein in Gene Editing
Recognition of Target DNA Sequence
The first step of Cas9 protein in gene editing is recognizing the target DNA sequence, relying on the cooperative function of the guide RNA and the PAM sequence. The guide RNA comprises two parts: one is a specific sequence complementary to the target DNA sequence (around 20 nucleotides), and the other is a scaffold sequence that binds with the Cas9 protein. Upon binding with the guide RNA, the Cas9 protein forms a Cas9-sgRNA complex. This complex continuously searches for DNA within cells, and when it encounters the target DNA sequence complementary to the guide RNA, a stable double-strand structure forms through base pairing between the guide RNA and target DNA. Concurrently, the Cas9 protein's PAM recognition domain identifies the PAM sequence downstream of the target DNA sequence. Only when both conditions are met can the Cas9-sgRNA complex stably bind to the target DNA site.
Complex Formation and Cleavage
Once the Cas9-sgRNA complex correctly binds to the target DNA site, the Cas9 protein undergoes significant structural changes. The originally closed Cas9 protein opens to form an open conformation suitable for DNA binding. At this point, the HNH nuclease domain and RuvC nuclease domain respectively position on the target and non-target strands, cleaving at specific locations. The HNH domain cleaves the 3' end of the target strand at the third nucleotide downstream of the area complementary to the guide RNA, while the RuvC domain cleaves the non-target strand approximately 3 nucleotides from the 5' end of the PAM sequence, ultimately leading to a double-strand break in DNA, creating a double-strand gap.
Cellular DNA Damage Repair Pathways Post-Cleavage
After a DNA double-strand break occurs, cells initiate their intrinsic DNA repair mechanisms to mend the damage. There are two primary repair pathways: non-homologous end joining (NHEJ) and homology-directed repair (HDR).
Non-homologous end joining is an error-prone repair pathway that does not require a template and directly joins the broken DNA ends. During this process, base insertions or deletions (Indel) often occur, leading to mutations in the gene sequence. Utilizing this characteristic, scientists can induce NHEJ repair through Cas9 protein cutting the target gene, causing the target gene to lose function and achieve gene knockout.
Homology-directed repair is an accurate repair pathway requiring a homologous template (usually a DNA fragment with the target gene modification sequence). In the presence of a homologous template, cells use it to accurately repair the broken DNA, enabling precise editing of genes, such as gene knock-in, point mutations, etc.
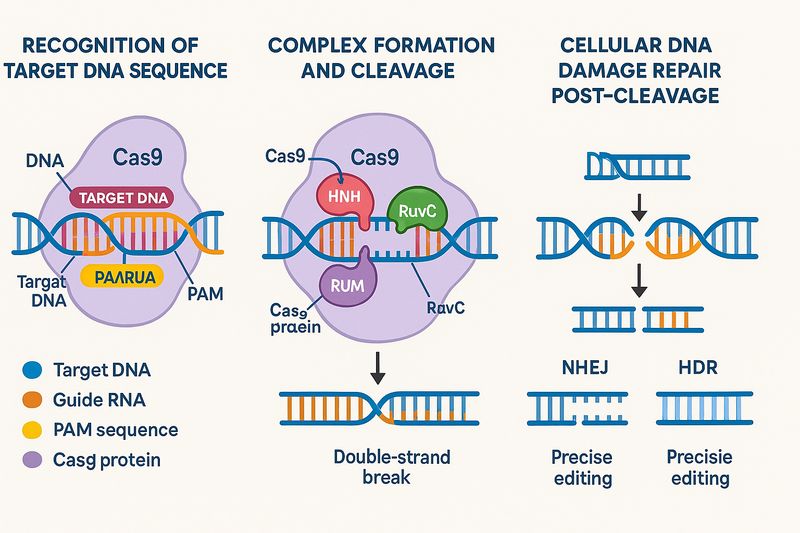 Fig3. Crispr-Cas9 Gene Edit Mechanism
Fig3. Crispr-Cas9 Gene Edit MechanismAdvantages and Limitations of Cas9 Protein
Advantages
Compared with other gene editing tools like zinc finger nucleases (ZFN) and transcription activator-like effector nucleases (TALEN), the Cas9 protein boasts several significant advantages.
Firstly, it offers a simple and convenient design. ZFN and TALEN require designing specific DNA-binding domains for each target sequence, a time-consuming, laborious, and costly process. Cas9 protein, on the other hand, only requires designing an sgRNA complementary to the target DNA sequence to achieve specific gene editing, significantly simplifying experimental operations, reducing technical barriers, and enabling more laboratories and researchers to easily engage in gene editing studies.
Secondly, it is cost-effective. The relatively simpler design of Cas9 protein results in reagent and material costs far lower than those of ZFN and TALEN, strongly supporting the widespread application of gene editing technology.
Furthermore, it offers high editing efficiency. The Cas9 protein can efficiently cleave target DNA sequences, inducing cellular repair mechanisms to achieve gene editing. It exhibits high editing efficiency across various cell types and organisms, yielding excellent experimental results both in cultured cells and live animals.
Additionally, Cas9 protein has a broad application range. It can be used for conventional gene editing operations such as gene knockout, gene knock-in, point mutations, etc., and can also be integrated with other functional modules to achieve gene expression regulation (e.g., activation or inhibition of gene expression), genome labeling, and more. Moreover, Cas9 protein can function in diverse organisms, including bacteria, yeast, plants, animals, and human cells, showcasing its robust versatility.
Limitations
Despite its numerous advantages, Cas9 protein also has limitations, with off-target effects being a primary concern. Partial base pairing may occur between the sgRNA and non-target DNA sequences, causing the Cas9-sgRNA complex to erroneously bind and cleave non-target sites, leading to off-target mutations. Off-target effects may pose potential safety risks, such as causing diseases like cancer, limiting the development of Cas9 protein in clinical applications.
Additionally, the delivery system for Cas9 protein is a pressing issue. In in vivo gene editing, how to safely and efficiently deliver the Cas9 protein and sgRNA to target cells is a crucial challenge. Current delivery methods include viral vector delivery and non-viral vector delivery, but viral vectors pose risks of insertional mutations and immune responses, while non-viral vector delivery efficiency is relatively low, making it challenging to meet practical application demands.
Furthermore, the size of the Cas9 protein is a limiting factor. The large molecular size may face capacity constraints in scenarios needing viral vector delivery, affecting its application.
Conclusion
The discovery and application of Cas9 protein as the "molecular scissors" in the field of gene editing mark a significant breakthrough in life science research. It not only provides scientists with an efficient and precise gene editing tool, advancing fundamental research development, but also demonstrates broad application prospects in medicine, agriculture, biotechnology, and other fields. Through Cas9 protein, we hope to cure genetic diseases, improve crop quality, develop novel biopharmaceuticals, etc., bringing tremendous benefits to societal development.
However, the Cas9 protein also presents limitations such as off-target effects and delivery system challenges that require ongoing research for improvements and optimization. Future research directions may include further enhancing Cas9 protein specificity to reduce off-target effects, developing safer and more efficient delivery systems for broader in vivo gene editing applications, and modifying and optimizing Cas9 protein for more features and better performance.
With ongoing scientific and technological advancements, we have reasons to believe that Cas9 protein will play an increasingly crucial role in the field of gene editing, contributing significantly to human exploration of life mysteries and solutions to health and environmental challenges.
Related Products & Services
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- ADC Target Protein
- Protein Engineering Services
- Protein Expression and Purification Services
- Stable Cell Line Services
Resource
- Cas9 Enzyme: A Revolutionary Tool Ushering in a New Era of Life Sciences
- Traditional Gene Manipulation Technologies and Zinc Finger Nucleases (ZFNs)
- TALENs: Precision Gene Editing for Research & Therapeutics
- The Big Three of Gene Editing: ZFNs, TALENs & CRISPR - Understand Their Differences and How to Choose
-
Unlocking the Power of Cas9: The Future of Genetic Science
-
Unlocking CRISPR-Cas9: The Gene Editing Revolution
-
CRISPR-Cas9: Revolutionizing Genetic Science!
-
ZNFs: The Precision Tools of Gene Editing
-
Why TALENs Still Beat CRISPR for Precision Gene Editing!
-
CRISPR vs. TALEN vs. ZFNs: The Ultimate Gene Editing Battle!
References
- Guo C, Ma X, Gao F, Guo Y. Off-target effects in CRISPR/Cas9 gene editing. Front Bioeng Biotechnol. 2023 Mar 9;11:1143157. doi: 10.3389/fbioe.2023.1143157. PMID: 36970624; PMCID: PMC10034092.
- Qiao, J., Li, W., Lin, S., Sun, W., Ma, L., & Liu, Y. (2019). Co-expression of Cas9 and single-guided RNAs in Escherichia coli streamlines production of Cas9 ribonucleoproteins. Communications Biology, 2(1), 1-6. https://doi.org/10.1038/s42003-019-0402-x
- Akinci, E., Hamilton, M. C., Khowpinitchai, B., & Sherwood, R. I. (2021). Using CRISPR to understand and manipulate gene regulation. Development (Cambridge, England), 148(9), dev182667. https://doi.org/10.1242/dev.182667
- Anna Dean. How CTX001 Therapy for Sickle Cell Disease Overcomes Many Challenges Associated with CRISPR Therapeutics. bilt.online
- Saini, H., Thakur, R., Gill, R., Tyagi, K., & Goswami, M. (2023). CRISPR/Cas9-gene editing approaches in plant breeding. GM Crops & Food, 14(1), 1. https://doi.org/10.1080/21645698.2023.2256930
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

