Cas9 Enzyme: A Revolutionary Tool Ushering in a New Era of Life Sciences
Introduction
Throughout the long journey of exploring life sciences, humanity has faced numerous significant challenges. These range from accurate analysis of gene functions and tackling complex genetic diseases, to breeding crop varieties with favorable traits. Each field has its own bottleneck awaiting breakthroughs. Traditional gene manipulation technologies, like Zinc Finger Nucleases (ZFNs) and Transcription Activator-Like Effector Nucleases (TALENs), have propelled the advancement of life sciences to a certain extent. However, they are fraught with issues such as complexity, high costs, and limited targeting efficiency, failing to meet the demands of modern life science research and applications.
In 2012, the advent of CRISPR-Cas9 gene-editing technology dramatically altered this landscape. As a key component of this technology, the Cas9 enzyme gained rapid prominence in life sciences for its precision, efficiency, and programmability, ushering in a new epoch of research and application. It functions as a "genetic scissor," precisely cutting and editing at specific genomic loci, providing an unprecedented tool to tackle numerous challenges within the life sciences.
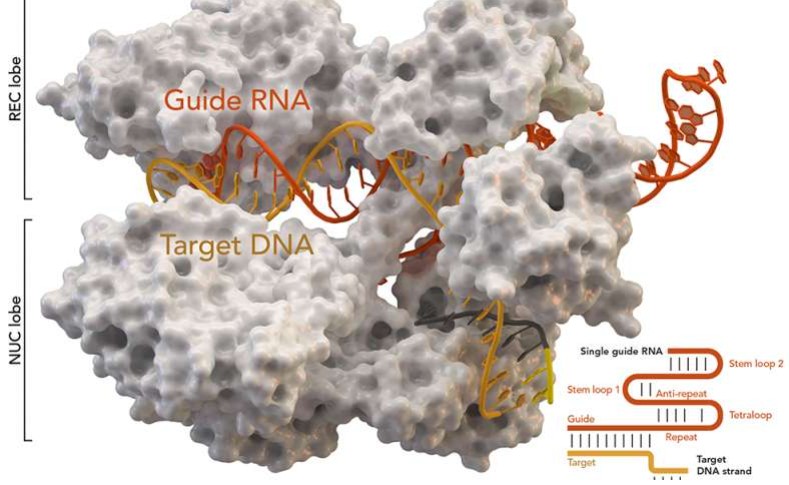 Fig1. CRISPR-associated protein Cas9
Fig1. CRISPR-associated protein Cas9Applications of Cas9 Enzyme in Basic Biological Research
Powerful Support for Gene Function Research
In gene function studies, constructing gene knockout cell lines and animal models is crucial. Scientists can understand gene functions and roles in life processes by observing phenotypic changes in cells or animals after specific genes are knocked out. Traditional gene knockout methods are time-consuming, labor-intensive, and inefficient. The emergence of the Cas9 enzyme has revolutionized this scenario.
With Cas9, scientists only need to design specific guide RNA (gRNA) to bind with sequences of the target gene. The Cas9 enzyme then cuts the DNA double strand accurately, triggering the cell's DNA repair mechanisms. Through Non-Homologous End Joining (NHEJ) or Homology-Directed Repair (HDR), genes can be knocked out, inserted, or replaced. For example, when studying tumor-related genes, scientists can use Cas9 to create specific gene knockout tumor cell lines to observe changes in cell proliferation, apoptosis, and migration, thereby clarifying the gene's role in tumor development.
In terms of animal model construction, Cas9 shows significant advantages. Traditional knockout mice models require complicated steps like embryonic stem cell targeting, taking months or even years. Cas9-mediated gene editing allows direct injection of Cas9 protein and gRNA into fertilized eggs for efficient gene editing, significantly shortening the time for animal model construction. Successfully, various knockout or knock-in animal models, such as mice, rats, and zebrafish, have been established, strongly supporting research on gene functions in development, immunity, and neural systems.
A Precise Tool for Gene Expression Regulation
Beyond knockout capabilities, Cas9 can regulate gene expression. By modifying Cas9 to lose its nuclease activity (dCas9) and fusing it with transcriptional activators or repressors, gene activation or repression can be achieved. This technique is known as CRISPR-activation (CRISPRa) and CRISPR-interference (CRISPRi).
In gene expression regulation studies, CRISPRa and CRISPRi can be used to screen key genes that regulate specific biological processes. For instance, in studying cell differentiation, researchers can activate candidate gene expression using CRISPRa to see if it prompts specific differentiation directions; using CRISPRi to inhibit candidate gene expression to observe whether cell differentiation is hindered. This high-throughput screening method quickly identifies key genes and regulatory networks involved in cell differentiation.
Furthermore, Cas9 can be applied in epigenetic modification studies. By fusing dCas9 with epigenetic modifier enzymes like DNA methyltransferases or histone modifiers, precise control over epigenetic modifications of specific gene regions can be achieved. This allows exploration of epigenetics' role in gene expression regulation and cell fate determination.
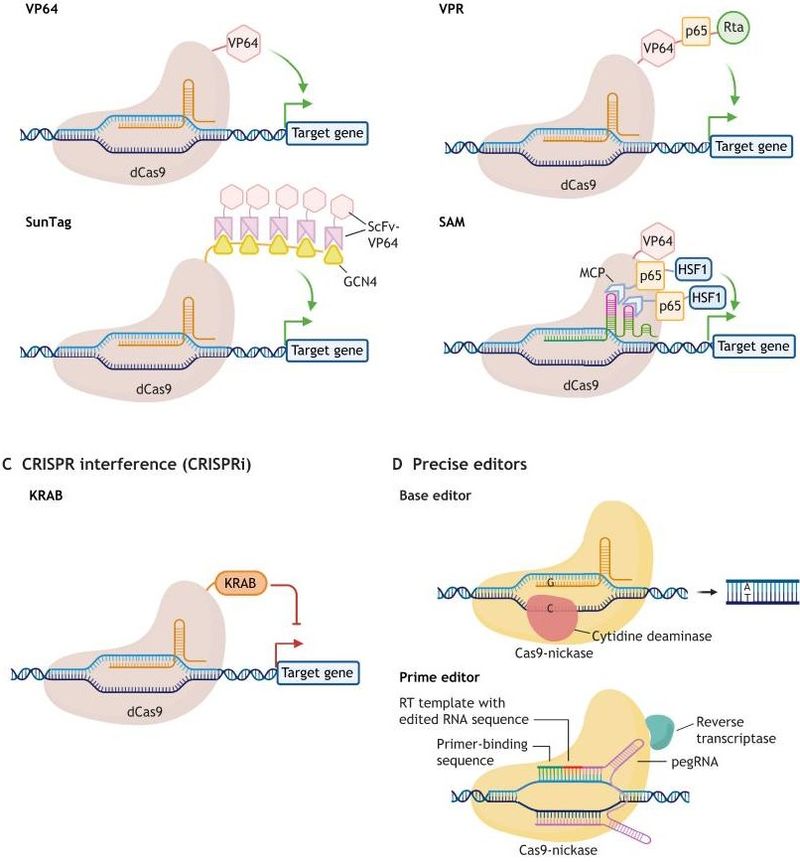 Fig2. The CRISPR toolbox
Fig2. The CRISPR toolboxCas9 Related Proteins
| Cat.No. | Product Name | Source | Species | Tag |
|---|---|---|---|---|
| CAS9-22S | Active Recombinant Full Length Streptococcus pyogenes serotype M1 type II CRISPR RNA-guided endonuclease Cas9 Protein, GFP-tagged | E.coli | Streptococcus pyogenes serotype M1 | GFP |
| Cas9 -121S | Recombinant CRISPR Cas9 protein | E.coli | Non | |
| cas9-12S | Active Recombinant Streptococcus pyogenes M1 cas9 Protein, His-tagged | Insect Cells | Streptococcus pyogenes M1 | His |
| cas9-11S | Recombinant Streptococcus pyogenes cas9 protein | E.coli | Streptococcus pyogenes | Non |
| cas9-12 | Recombinant Cas9 Nuclease Protein | E.coli | Non | |
| cas9-85H | Recombinant bacterial Cas9 protein, His-tagged | E.coli | Streptococcus pyogenes serotype M1 | His |
| cas9-01S | Recombinant S. pyogenes Cas9 Protein, GFP-Labeled | E.coli | Streptococcus pyogenes |
Case Studies of Cas9 Enzyme Advancing Medicine
New Hope for Genetic Diseases Gene Therapy
Genetic diseases, like sickle cell anemia and Duchenne muscular dystrophy, arise from gene defects. Traditional treatments cannot cure them, causing enormous suffering. Cas9-mediated gene editing brings hope for treating these genetic diseases.
Sickle Cell Anemia Treatment Research
Sickle cell anemia stems from a mutation in the hemoglobin β gene (HBB), leading to misshaped red blood cells causing hemolytic anemia and vascular blockage. Using Cas9, scientists can edit patients' hematopoietic stem cells to repair the mutated HBB gene. The process involves extracting these cells, cutting the mutated gene with Cas9 and gRNA, introducing normal gene sequences through HDR, then amplifying and reinfusing the edited cells back into patients to produce normal red blood cells.
Currently, multiple clinical trials are underway. For instance, the CRISPR-based therapy CTX001, developed by CRISPR Therapeutics and Vertex Pharmaceuticals, targets sickle cell anemia and β-thalassemia. Clinical trials have shown significant improvement in patients, reduced transfusion needs, and increased hemoglobin levels, indicating promising therapeutic effects.
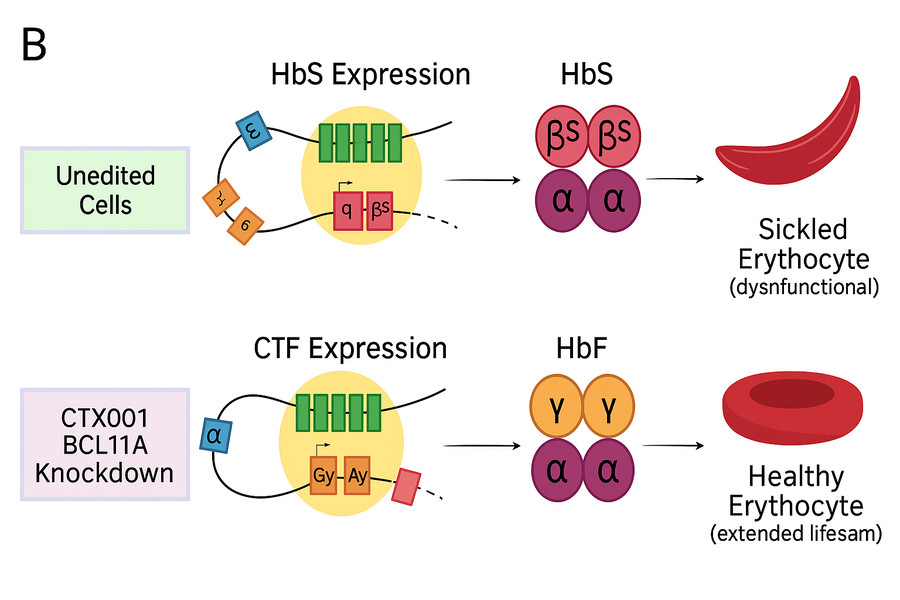 Fig3. Effect of CTX001-mediated BCL11A Knockdown
Fig3. Effect of CTX001-mediated BCL11A KnockdownProgress in Duchenne Muscular Dystrophy Treatment
Duchenne muscular dystrophy, an X-linked recessive muscle degenerative disorder, arises from dystrophin gene (DMD) defects causing muscle cell damage. Cas9 can edit the DMD gene, repairing missing or mutated exons to restore dystrophin protein expression.
Scientists design gRNA targeting specific DMD gene exons, using Cas9 to cut the gene, inducing NHEJ repair to skip mutated exons, allowing the gene to encode partially functional dystrophin. Animal models show this method improves muscle function and increases dystrophin expression. Though not yet in large-scale trials, it holds potential for treating Duchenne muscular dystrophy.
New Strategies for Cancer Treatment
Aside from genetic disorders, Cas9 presents broad prospects in cancer treatment. Cancer arises from mutations causing abnormal cell growth and differentiation; Cas9 can target oncogenes or activate tumor suppressor genes to inhibit tumor growth.
For example, in CAR-T cell therapy, Cas9 edits T cells to knock out the PD-1 gene, boosting T cell anti-tumor activity and prolonging its survival. Edited CAR-T cells have shown better treatment effects against certain cancer types in trials.
Additionally, Cas9 screens tumor treatment targets. Whole-genome CRISPR screenings in tumor cells quickly identify essential genes for tumor growth, offering drug development targets.
Car-T Cell Therapy Target Proteins
| Cat.No. | Product Name | Source | Species | Tag |
|---|---|---|---|---|
| TNFRSF17-552H | Active Recombinant Human TNFRSF17, Fc-tagged, Biotinylated | Human Cells | Human | Fc |
| CD19-3309HP | Active Recombinant Human CD19 protein, His-tagged, R-PE labeled | HEK293 | Human | His |
| EGFR-692HA | Active Recombinant Human EGFR protein, Fc-tagged, APC labeled | HEK293 | Human | Fc |
| CD19-3308H | Active Recombinant Human CD19, Fc tagged | HEK293 | Human | Fc |
| CD274-131C | Active Recombinant Cynomolgus/Rhesus CD274 protein(Met1-Thr239), Fc-tagged | HEK293 | Cynomolgus/Rhesus | Fc |
| Cd38-536M | Active Recombinant Mouse Cd38, His-tagged | Mammalian Cells | Mouse | His |
| PDCD1-172H | Active Recombinant Human PDCD1, no tag | HEK293 | Human | Non |
The Potential of Cas9 Enzyme in Agriculture
Cultivating Superior Crop Varieties
In agriculture, cultivating crops with desirable traits is key to boosting yield, improving quality, and enhancing stress resistance. Traditional breeding relies on natural mutations and hybrid screenings, which are lengthy and inefficient, hampered by species reproductive isolation. Cas9-mediated gene editing precisely edits crop target genes, breaking species barriers to breed superior varieties quickly.
Enhancing Disease and Pest Resistance
Diseases and pests threaten agricultural production, causing global losses annually. Using Cas9, scientists can edit crop genes related to disease and pest resistance, augmenting crops' capabilities. For example, in rice, editing blast disease resistance genes yields resistant varieties; in wheat, editing powdery mildew resistance genes boosts resistance.
Improving Crop Quality
Cas9 can also enhance crop quality, improving nutritional value, flavor, and shelf life. In tomatoes, editing genes controlling fruit ripening extends shelf life; in rice, editing lysine synthesis genes enhances nutritional value.
Boosting Stress Resistance
Facing climate-induced stressors like drought and salinity, editing crops' stress-related genes, such as drought response or salt tolerance genes, fosters varieties with stronger resilience, increasing yield and survival in harsh environments.
Table1. Role of CRISPR/Cas9 gene-editing technology on different agricultural crops with enhanced or improved trait.
| Crop | Enhanced or Improved trait by CRISPR | Target Gene |
|---|---|---|
| Arabidopsis thaliana | Drought resistance | AtOST2 |
| Arabidopsis thaliana | Salt resistance | AtWRKY and AtWRKY4 |
| Arabidopsis thaliana | Metal stress tolerance | Atoxp1 |
| Beta vulgaris | Severe curly top virus | IR |
| Brassica oleracea | Mosaic virus | CP |
| Brassica napus | Herbicide resistance | OsALS |
| Capsicum frutiscens | Leaf curl virus | C1/C4 + V1/V2 |
| Cucumis sativus | Mosaic virus | ORF1a, ORF 3a, 3 0UTR |
| Glycine max | Salt resistance | GmDrb2a and GmDrb2b |
| Gossypiun herbaceum | Leaf curl Multan virus | Rep + IR |
| Gossypiun herbaceum | Leaf curl virus and beta satellite | Rep |
| Gossypium hirsutum | Heat resistance | GhPGF and GhCLA1 |
| Manihot esculenta | Cyanide reduction | CYP79D1 and CYP79D2 |
| Manihot esculenta | Herbicide resistance | OsALS |
| Medicago sativa L. | Biomass yield and quality | Msfta1 |
| Musa sp. | Streak virus | ORF1, 2, 3 |
| Oryza sativa | Salt resistance | OsDST |
| Oryza sativa | Salt resistance | OsSPL10 |
New Directions in Livestock Breeding
In livestock breeding, Cas9 holds immense potential. Gene editing can enhance livestock growth, meat quality, and disease resistance. For instance, in pigs, editing growth hormone receptor gene boosts growth and feed conversion rates; in chickens, editing antiviral genes strengthens disease resistance.
Current Status and Prospects for Cas9 Enzyme Commercialization
Industry Deployment
With rapid Cas9 advancements, more companies enter this field. Industry leaders like CRISPR Therapeutics, Editas Medicine, and Intellia Therapeutics focus on developing gene therapy drugs for genetic diseases and cancers, conducting clinical trials. Agricultural firms like Bayer and Monsanto actively research crop breeding with Cas9 technology.
Growth Trends in Market Scale
Market research forecasts rapid growth in the global gene editing market. As Cas9 expands its applications in medicine and agriculture, the market will reach billions by 2025. Gene therapy will be the fastest-growing segment, propelled by ongoing trials and drug approvals.
Opportunities and Challenges
Opportunities
- Policy Support: Many countries recognize gene editing as a key frontier, offering policies to support its research and application. The U.S. FDA approved several Cas9-based gene therapy trials, while the EU advances its development actively.
- Technological Innovation: In-depth Cas9 studies yield new variants and improved editing technologies like base and prime edits, enhancing precision and efficiency, broadening applications.
- Market Demand: With aging populations and rising genetic disease rates, gene therapy demand increases. In agriculture, population growth and food security drive demand for superior crops, providing market space for Cas9 commercialization.
Challenges
- Ethical Issues: Gene editing involves human gene modifications, raising ethical concerns about safety, fairness, and genetic library impact. Comprehensive ethical regulations must ensure responsible use.
- Technical Hurdles: Despite progress, Cas9 faces challenges like off-target effects, in vivo editing efficiency limits, and long-term safety. Further research is needed to enhance safety and efficacy.
- Regulatory Environment: Varying regulations across countries hinder commercialization and global roll-out. Unified standards are needed for international development.
Conclusion
As the core of CRISPR-Cas9 technology, Cas9 enzyme has shown vast application potential in basic biology, medicine, and agriculture, heralding a new era in life sciences. It provides unprecedented tools for researchers, offering new hope for solving major issues like genetic disorders and food security.
Although Cas9 commercialization faces ethical, technical, and regulatory challenges, ongoing research, technological advances, and improved regulatory systems will resolve these. Looking ahead, Cas9 is poised for more profound impacts across fields like synthetic biology, regenerative medicine, and environmental protection. It will drive life sciences from theory to practical application, creating a better future. We can confidently assert that Cas9 will be a milestone in life sciences, leading us toward a more precise, efficient, sustainable biotech era.
Related Products & Services
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- ADC Target Protein
- Protein Engineering Services
- Protein Expression and Purification Services
- Stable Cell Line Services
Resource
- Cas9 Protein: Unveiling the "Molecular Scissors" of Gene Editing
- Traditional Gene Manipulation Technologies and Zinc Finger Nucleases (ZFNs)
- TALENs: Precision Gene Editing for Research & Therapeutics
- The Big Three of Gene Editing: ZFNs, TALENs & CRISPR - Understand Their Differences and How to Choose
-
Unlocking the Power of Cas9: The Future of Genetic Science
-
Unlocking CRISPR-Cas9: The Gene Editing Revolution
-
CRISPR-Cas9: Revolutionizing Genetic Science!
-
ZNFs: The Precision Tools of Gene Editing
-
Why TALENs Still Beat CRISPR for Precision Gene Editing!
-
CRISPR vs. TALEN vs. ZFNs: The Ultimate Gene Editing Battle!
References
- Guo C, Ma X, Gao F, Guo Y. Off-target effects in CRISPR/Cas9 gene editing. Front Bioeng Biotechnol. 2023 Mar 9;11:1143157. doi: 10.3389/fbioe.2023.1143157. PMID: 36970624; PMCID: PMC10034092.
- Qiao, J., Li, W., Lin, S., Sun, W., Ma, L., & Liu, Y. (2019). Co-expression of Cas9 and single-guided RNAs in Escherichia coli streamlines production of Cas9 ribonucleoproteins. Communications Biology, 2(1), 1-6. https://doi.org/10.1038/s42003-019-0402-x
- Akinci, E., Hamilton, M. C., Khowpinitchai, B., & Sherwood, R. I. (2021). Using CRISPR to understand and manipulate gene regulation. Development (Cambridge, England), 148(9), dev182667. https://doi.org/10.1242/dev.182667
- Anna Dean. How CTX001 Therapy for Sickle Cell Disease Overcomes Many Challenges Associated with CRISPR Therapeutics. bilt.online
- Saini, H., Thakur, R., Gill, R., Tyagi, K., & Goswami, M. (2023). CRISPR/Cas9-gene editing approaches in plant breeding. GM Crops & Food, 14(1), 1. https://doi.org/10.1080/21645698.2023.2256930
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

