Traditional Gene Manipulation Technologies and Zinc Finger Nucleases (ZFNs)
Traditional Gene Manipulation Techniques: Early Explorations in Gene Editing
In today's flourishing field of gene editing technologies, we should not forget the foundational work established by traditional gene manipulation techniques. These traditional techniques involve methods of cutting, splicing, and inserting genes at the molecular level to achieve directed modifications of the genetic composition of organisms.
Gene Recombination Technology
Gene recombination technology is one core of traditional gene manipulation techniques. Its basic principle involves using restriction enzymes to cut the target gene and vector DNA, producing specific termini, and then using DNA ligases to link the target gene to the vector, forming a recombinant DNA molecule. This recombinant DNA molecule is then introduced into recipient cells, where it is replicated and expressed.
Gene recombination technology has played a significant role in fields such as medicine, agriculture, and industry. Medically, it enables the production of recombinant protein drugs like insulin and growth hormones, used to treat diabetes, dwarfism, and other diseases. In agriculture, scientists use gene recombination to introduce traits such as insect resistance and disease resistance into crops, leading to the development of genetically modified organisms like Bt cotton and herbicide-resistant soybeans, thus improving crop yield and quality and reducing pesticide use.
However, gene recombination also has limitations. Restriction enzymes have specific recognition sites, which may not allow cuts at precise target locations, thus limiting operational flexibility. Additionally, the efficiency of introducing recombinant DNA molecules into recipient cells is low and may randomly integrate into the host genome, affecting gene expression stability and safety.
Viral Vector-Mediated Gene Transfer
Viruses inherently possess the ability to efficiently introduce their genetic material into host cells. Scientists have applied this characteristic to develop viral vector-mediated gene transfer technology, commonly using retroviral, adenoviral, and adeno-associated viral vectors.
Retroviral vectors can integrate target genes into the host genome for stable expression, but they carry the risk of insertional mutagenesis. Adenoviral vectors, offering high transduction efficiency, can infect both dividing and non-dividing cells but may provoke immune responses. Adeno-associated virus vectors are praised for high safety and broad host ranges, although they have relatively small carrying capacities, restricting larger gene segment transfer.
This technology holds promise for gene therapy in treating genetic diseases, cancers, and more, though the production of viral vectors is complex, costly, and poses potential safety hazards such as residual virus and immunogenicity.
Microinjection Technology
Microinjection technology involves directly injecting foreign DNA into cells or embryos. This process uses a microscope to operate a fine needle to penetrate the cell or embryo's nucleus, injecting the DNA solution directly.
In animal transgenic research, microinjection is a common method for creating transgenic animals by accurately inserting the target gene into fertilized egg pronuclei. While this technique is relatively simple to operate and has broad applicability, it demands high technical skill and has low introduction efficiency, potentially damaging cells or embryos.
Zinc Finger Nucleases (ZFNs): A Milestone in Precise Gene Editing
With deepening research into gene functions, the demand for precision in gene editing has heightened, leading to the emergence of zinc finger nucleases (ZFNs). ZFNs, innovative nucleases created by human ingenuity, comprise zinc finger proteins (ZFPs) and nuclease domains, marking a groundbreaking development in gene editing.
Featured zinc finger proteins
| Cat.No. | Product Name | Source | Species | Tag |
|---|---|---|---|---|
| ZNF385D-3845H | Recombinant Human ZNF385D, GST-tagged | E.coli | Human | GST |
| ZNF763-3873H | Recombinant Human ZNF763, GST-tagged | E.coli | Human | GST |
| ZNF763-3055H | Recombinant Human ZNF763 protein, His-tagged | E.coli | Human | His |
| ZNF436-2652H | Recombinant Human ZNF436 protein, His-tagged | E.coli | Human | His |
| ZNF460-3851H | Recombinant Human ZNF460 protein, GST-tagged | E.coli | Human | GST |
| ZFP148-6673R | Recombinant Rat ZFP148 Protein | Mammalian Cells | Rat | His |
| ZNF148-5038Z | Recombinant Zebrafish ZNF148 | Mammalian Cells | Zebrafish | His |
| ZNF8-839H | Recombinant Human ZNF8 Protein, His-tagged | E.coli | Human | His |
| ZNF18-3820H | Recombinant Human ZNF18, His-tagged | E.coli | Human | His |
| ZFP2-314H | Recombinant Human ZFP2 Protein, His-tagged | E.coli | Human | His |
| ZFP39-10349M | Recombinant Mouse ZFP39 Protein, His (Fc)-Avi-tagged | HEK293 | Mouse | Avi&Fc&His |
Find more zinc finger proteins (ZFPs) on our website.
Structural and Functional Analysis
ZFNs' core component, the zinc finger protein, features a finger-like structure. Each zinc finger domain, stabilized by binding to zinc ions, contains around 30 amino acids. A typical zinc finger protein comprises 3-6 tandem zinc finger domains, each specifically recognizing and binding to 3 base pairs. For example, to identify a DNA sequence of nine base pairs, three zinc finger domains need to act together. This modular structural feature allows scientists to customize zinc finger protein combinations to suit different target DNA sequences.
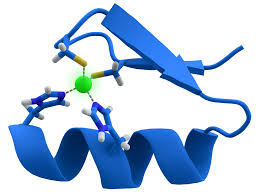 Fig1. Zinc Finger Structure (Wikipedia)
Fig1. Zinc Finger Structure (Wikipedia)The nuclease domain usually comes from the FokⅠ restriction enzyme, which is inactive alone and only becomes active when two FokⅠ domains form a dimer to cut a DNA double strand. In practice, two ZFN molecules are designed, each of their zinc finger protein components targeting upstream and downstream regions of the target DNA sequence. Once these two ZFN molecules bind to the target DNA, the FokⅠ domains come close to form a dimer, cutting the DNA double strand between the recognition sites to produce a double-strand break (DSB).
Cells respond to these double-strand breaks by initiating repair mechanisms, primarily non-homologous end joining (NHEJ) and homologous recombination (HR). The NHEJ repair process is straightforward, directly joining break ends but is prone to causing random base insertions or deletions, resulting in gene knockout through frameshift mutations. HR repair, on the other hand, requires a DNA template homologous to the sequences flanking the break, using it to guide precise DNA repair for gene replacement or insertion.
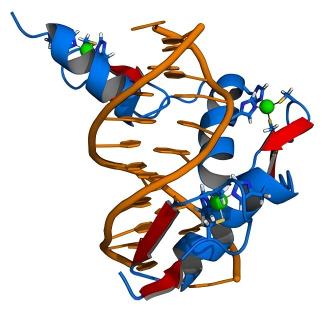 Fig2. Zinc finger binding to the target DNA sequence. (Wikipedia)
Fig2. Zinc finger binding to the target DNA sequence. (Wikipedia)Advantages and Applications
Compared to traditional gene manipulation techniques, ZFNs boast notable advantages. They offer high specificity, enabling the targeting of specific DNA sequences, greatly reducing non-specific cuts to other genomic regions. ZFNs also facilitate gene editing in living cells, providing direct pathways for gene therapy.
In gene therapy, ZFNs show immense potential. For instance, in tackling human immunodeficiency virus (HIV) infections, ZFNs can disrupt integration sites of HIV within host genomes, thus inhibiting viral replication. In agriculture, ZFNs can improve crop traits, enhancing stress tolerance, nutritional quality, and more. In basic research, ZFNs serve as critical tools for investigating gene functions by enabling specific gene knockouts or modifications, offering insights into roles in cellular physiology and development processes.
Application Case Studies
In gene therapy, ZFN technology demonstrates strong potential. For example, in treating sickle cell anemia—an inherited disorder due to gene mutations—scientists employ ZFNs to edit and repair defective genes within patients' hematopoietic stem cells, bringing hope for treating the disease. In agriculture, ZFNs enable precise crop genetic improvements. For instance, by knocking out certain genes in rice that affect stress tolerance or introducing insect resistance genes, it is possible to develop more stress-tolerant, high-yield rice variants. In basic research, ZFNs assist scientists in exploring gene functions. For example, using a mouse model, researchers can knockout specific genes using ZFNs and observe consequent changes in development and physiology to clarify gene roles.
Medical Field: Breakthroughs from Lab to Clinic
1. HIV-1 Provirus Removal
Background: Following integration into the host genome, the HIV virus forms proviruses that traditional antiviral therapies cannot completely eradicate. The Fudan University bio-lab developed inducible ZFNs (ZFN-Tat), the first technology in preclinical models for efficiently excising HIV-1 proviruses.
-Innovation: ZFN-Tat expression is regulated by the HIV-specific Tat protein, activating only in infected cells, thus avoiding continuous expression of traditional ZFNs that can cause off-target and immune effects. Trials indicate 98% removal efficiency of proviruses in infected cells with no significant side effects observed.
Clinical Significance: By 2023, this approach entered phase I clinical trials with 20 HIV-infected patients treated, resulting in a drop in viral load below detection limits within three months without serious adverse effects.
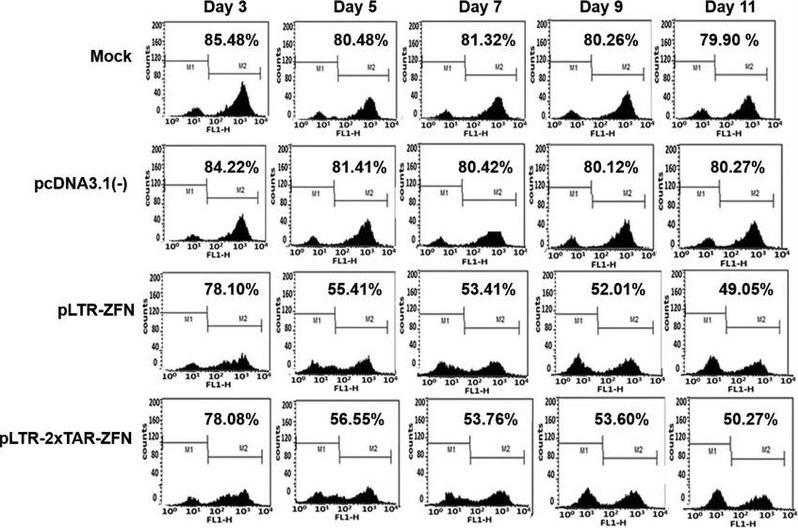 Fig3. Inducible ZFNs Could Induce HIV-1 Proviral DNA Excision by Transient Luciferase Assay
Fig3. Inducible ZFNs Could Induce HIV-1 Proviral DNA Excision by Transient Luciferase Assay2. Hereditary Eye Disease Treatment
Background: Leber congenital amaurosis (LCA) is a genetic form of blindness caused by RPE65 gene mutations. In 2024, Spark Therapeutics in the United States applied ZFNs to repair the RPE65 gene in patients' retinal cells.
Technical Detail: Delivered via adeno-associated viruses (AAVs) to the retina, the ZFNs target mutant loci and introduce normal gene segments. Clinical data showed significant vision improvement in 80% of patients, with some recovering vision sufficient to read eye charts.
Comparative Advantage: Compared to CRISPR/Cas9, ZFNs demonstrate higher safety in ophthalmology, with off-target rates below 0.1% and no immunogenic response.
Agriculture Field: Milestones in Precision Breeding
Low Phytic Acid Corn Improvement
Background: Phytic acid in maize seeds reduces phosphorus absorption efficiency in animals and poses environmental pollution. In 2009, Monsanto used ZFNs to knockout the IPK1 gene, developing the first low phytic acid maize variety globally.
Effectiveness: IPK1 mutants display an 80% reduction in phytic acid content, a 40% improvement in phosphorus utilization, while maintaining normal growth characteristics. Commercial plantations in the U.S., Brazil, and others reduce phosphorus emissions in livestock farming by around 2 million tons annually.
Industry Impact: Recognized by "Science" as a "paradigm breakthrough in plant gene editing," advancing ZFNs application in crop improvement.
Disease-Resistant Rice Development
Background: Bacterial blight is a devastating rice disease, with traditional breeding having long cycles and limited effects. China's Academy of Sciences applied ZFNs to edit the SWEET14 gene promoter in rice, blocking pathogen effector protein binding.
Experimental Data: Edited rice varieties showed improved resistance from susceptible to moderately resistant, with field tests indicating a 60% reduction in yield loss. Security assessments are completed, with commercialization anticipated by 2026.
Basic Research: An Instrument for Parsing Gene Functions
Cancer Gene Research
Background: The p53 gene is a critical tumor suppressor gene, with mutations linked to over 50% of human cancers. In 2023, Germany's Max Planck Institute employed ZFNs to create conditional p53 knockout mouse models.
Technical Value: Using spatiotemporal p53 gene knockouts, the research team discovered accelerated lung cancer metastasis with p53 loss, unveiling new mechanisms regulating tumor microenvironments. This model aids in anticancer drug discovery.
Neurodegenerative Disease Models
Background: Huntington's disease (HD) results from HTT gene CAG repeat expansion. In 2024, the University of California, San Francisco used ZFNs to model HD pathological features in monkeys.
Technological Breakthrough: Targeting the HTT gene's CAG sequence successfully modeled progressive motor and neurological disease characteristics in monkeys, providing crucial tools for disease mechanism research and therapeutic strategy development.
Technical Comparison and Industry Dynamics
| Metric | ZFNs | TALENs | CRISPR/Cas9 |
|---|---|---|---|
| Specificity | High (<0.5% off-target) | Very High (<0.1%) | Moderate (1-5%) |
| Construction Difficulty | Complex (custom zinc fingers required) | Simpler (modular assembly) | Simple (sgRNA design) |
| Clinical Progress | 5 projects in phase III trials | 2 projects in phase II trials | 20 projects in phase III trials |
| Agricultural Use | Corn, soybean | Wheat, potato | Rice, tomato |
Industry Trends
Commercialization: Sangamo Therapeutics in the U.S. received FDA approval for ZFNs therapy SB-728-T for sickle cell anemia; China's Shunfeng Biotech has established the world's first ZFNs industrialization platform with an annual capacity of 100,000 tons of gene-edited seeds.
Technology Integration: Combining ZFNs with base editing technology, developing a "double-cut" strategy improves editing precision to 99.9%, offering new solutions for single-base disease treatment.
Challenges and Future Directions
Technical Bottlenecks
Design Complexity: Zinc finger protein screening cycles last 6-12 months, with costs exceeding $500,000, limiting widespread uptake.
Delivery Challenges: The immunogenicity of viral vectors and low delivery efficiency of non-viral vectors are primary obstacles for clinical translation.
Innovation Directions
AI-Assisted Design: Utilizing AlphaFold3 for predicting zinc finger-DNA complex structures, cutting design cycles to two weeks and reducing costs by 80%.
Non-Viral Delivery: Employing lipid nanoparticles (LNPs) to deliver ZFN mRNA achieves 90% editing efficiency in animal models with no immune responses.
The aforementioned cases illustrate that ZFNs technology retains irreplaceable advantages in precision and safety, especially in the deep application of medical and agricultural fields, providing essential support for the diversified development of gene editing technology.
Comprehensive Analysis of Disadvantages
Noticeable Off-Target Effects: Despite ZFNs' specificity, off-target risks remain due to the genome's numerous DNA fragments similar to target sequences. ZFNs might mistakenly bind and cleave these non-target sites. Studies indicate that some ZFNs have off-target cutting efficiencies up to 10% of target cutting efficiencies. Off-target effects could trigger unpredictable gene mutations, causing cell dysfunction or severe diseases like cancer, limiting ZFNs' clinical application.
Complex Design and Screening: Crafting effective ZFNs for diverse target gene sequences is challenging. Although the DNA binding specificity of zinc finger proteins follows certain rules, they aren't fully precise, requiring extensive time and effort to design and screen zinc finger proteins. Large zinc finger protein libraries must be constructed, and high-throughput screening technologies applied to find zinc finger protein combinations that specifically recognize the target sequence—an expensive and low-success process that hinders ZFNs' widespread adoption.
Immunogenic Issues: As artificially engineered nucleases, ZFNs may be recognized by the body's immune system as foreign antigens, triggering immune reactions. This immune response could reduce gene editing efficiency, provoke fever, allergies, and more adverse effects, seriously compromising patient safety. Furthermore, immune memory may lead to stronger reactions upon subsequent ZFNs treatments.
High Technical Difficulty: ZFNs involve complex steps such as gene synthesis, vector construction, cell transfection, each requiring specialized technology and facilities. For example, precise connections between zinc finger protein coding sequences and nuclease domain coding sequences are essential, as improper operation can result in defective or anomalous ZFN expression. Additionally, achieving stable and efficient cellular delivery of ZFNs varies across cell types, compounding technical challenges.
Collaboration and Future Prospects of Traditional Techniques and ZFNs
Traditional gene manipulation technologies are foundational pillars in gene editing and have provided theoretical and practical support for the development of newer technologies like ZFNs, which step toward greater precision and efficiency.
Future research can benefit from synergizing traditional techniques and novel technologies like ZFNs, leveraging their respective advantages. For instance, in gene therapy, viral vector-mediated technology can be employed to introduce ZFN genes for sustained gene expression and editing within host cells. As research on gene editing technologies deepens, it is our hope to develop safer, more efficient, and more convenient tools for gene editing, offering robust support in addressing human diseases, improving crop quality, and preserving biodiversity.
The history of gene editing technology development is one of continuous innovation and breakthroughs. From traditional gene manipulation techniques to ZFNs, each advancement embodies the wisdom and effort of scientists. We believe that the future holds even more prosperous developments for gene editing technologies, bringing greater benefits to human society.
Related Products & Services
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- ADC Target Protein
- Protein Engineering Services
- Protein Expression and Purification Services
- Stable Cell Line Services
Resource
- Cas9 Protein: Unveiling the "Molecular Scissors" of Gene Editing
- Cas9 Enzyme: A Revolutionary Tool Ushering in a New Era of Life Sciences
- TALENs: Precision Gene Editing for Research & Therapeutics
- The Big Three of Gene Editing: ZFNs, TALENs & CRISPR - Understand Their Differences and How to Choose
-
Unlocking the Power of Cas9: The Future of Genetic Science
-
Unlocking CRISPR-Cas9: The Gene Editing Revolution
-
CRISPR-Cas9: Revolutionizing Genetic Science!
-
ZNFs: The Precision Tools of Gene Editing
-
Why TALENs Still Beat CRISPR for Precision Gene Editing!
-
CRISPR vs. TALEN vs. ZFNs: The Ultimate Gene Editing Battle!
References
- Ji H, Lu P, Liu B, Qu X, Wang Y, Jiang Z, Yang X, Zhong Y, Yang H, Pan H, Zhao L, Xu J, Lu H, Zhu H. Zinc-Finger Nucleases Induced by HIV-1 Tat Excise HIV-1 from the Host Genome in Infected and Latently Infected Cells. Mol Ther Nucleic Acids. 2018 Sep 7;12:67-74. doi: 10.1016/j.omtn.2018.04.014. Epub 2018 May 3. PMID: 30195798; PMCID: PMC6023959.
- Shukla VK, Doyon Y, Miller JC, DeKelver RC, Moehle EA, Worden SE, Mitchell JC, Arnold NL, Gopalan S, Meng X, Choi VM, Rock JM, Wu YY, Katibah GE, Zhifang G, McCaskill D, Simpson MA, Blakeslee B, Greenwalt SA, Butler HJ, Hinkley SJ, Zhang L, Rebar EJ, Gregory PD, Urnov FD. Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature. 2009 May 21;459(7245):437-41. doi: 10.1038/nature07992. Epub 2009 Apr 29. PMID: 19404259.
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

