The Big Three of Gene Editing: ZFNs, TALENs & CRISPR - Understand Their Differences and How to Choose
Gene editing is a technology that allows for the modification of life's source code, with ZFNs, TALENs, and CRISPR being the three core tools for this task. This article will trace the history of these three generations of technology in an accessible way and provide an in-depth comparison of their respective strengths and weaknesses.
Gene Editing Tools: Rewriting the Book of Life – From "Heavy Tomes" to "Smart Editors"
Imagine life's blueprint—DNA—as a vast book written with 3 billion letters (base pairs). Genetic mutations are like typos or erroneous paragraphs in this book, potentially leading to disease or other issues. Gene editing technology is the "ultimate revision tool" that allows us to accurately find and correct these errors.
Over the past few decades, scientists have successively developed three main "editing tools": ZFNs, TALENs, and CRISPR. Each has its own merits and together they have propelled the rapid advancement of biotechnology. Understanding their evolution, differences, and applicable scenarios is crucial for investors, cross-disciplinary collaborators, and anyone interested in cutting-edge technology. This article will delve into these "Big Three of Gene Editing" and reveal how to make informed choices for specific projects.
Historical Evolution: The Innovation from "Cumbersome" to "Agile"
The development of gene editing technology is a history of evolution pursuing higher efficiency, lower cost, and greater precision.
First Generation: ZFNs – The "Precision Machine Tool" that Pioneered the Era
- Emergence: 1990s to early 2000s.
- Working Principle: ZFNs (Zinc Finger Nucleases) were the first tool to achieve targeted gene editing. They also consist of two parts: a DNA-binding domain (zinc finger protein) and a cleavage domain (FokI endonuclease). Zinc finger proteins are modules that can recognize specific three-base pair sequences. By combining multiple zinc finger modules, longer DNA sequences can be targeted.
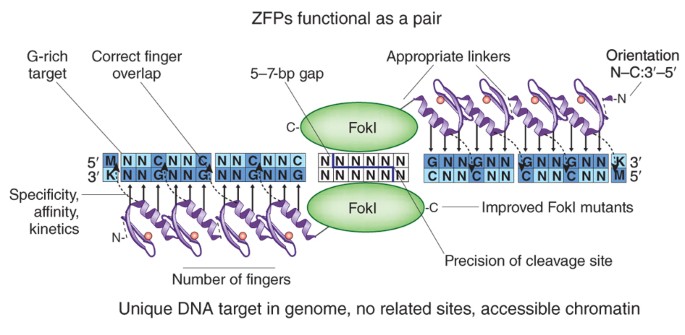 Fig1. Zinc-finger nucleases (Isalan, M. 2011)
Fig1. Zinc-finger nucleases (Isalan, M. 2011)- Historical Significance: The advent of ZFNs demonstrated the feasibility of targeted genome editing, making it the true pioneer. It achieved the breakthrough from "0 to 1" and was successfully applied in early basic research and clinical trials (e.g., the first gene editing therapy to enter clinical trials was based on ZFNs).
- The "Cumbersome" Aspect: Its biggest bottleneck was its extremely complex design. Zinc finger modules influence each other (context-dependent effects), the success rate of recognizing specific triplets isn't 100%, and the assembly process is tedious, time-consuming, and costly. It was like a precision machine tool requiring manual tuning by top craftsmen—powerful but low-yield, expensive, and difficult to popularize.
Second Generation: TALENs – The Modular "Precision Scalpel"
- Emergence: Post-2009.
- Working Principle: The emergence of TALENs (Transcription Activator-Like Effector Nucleases) was a major upgrade to ZFNs. Its DNA-binding domain comes from a plant bacterium (TALE protein). Its revolutionary aspect lies in high modularity: each TALE unit, like a Lego brick, specifically recognizes a single base (A, T, C, or G), and the units hardly interfere with each other.
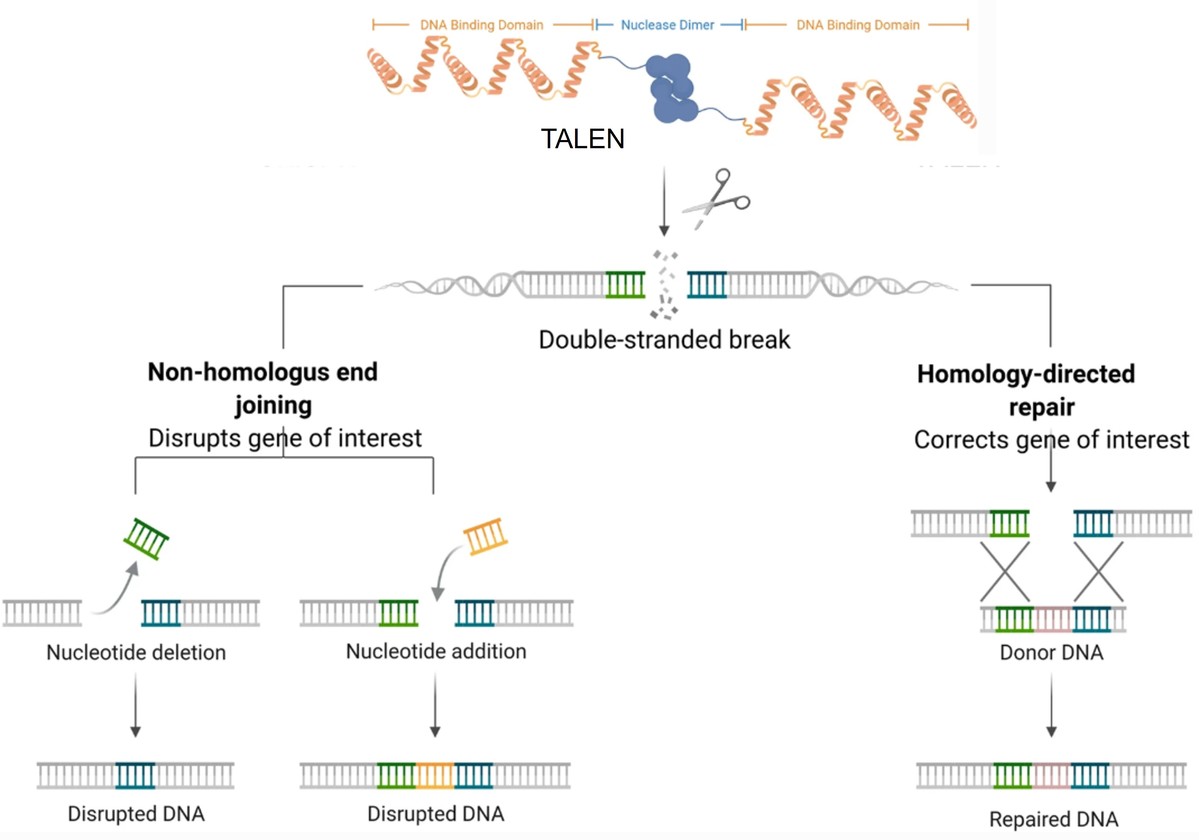 Fig2. Talen working mechanism
Fig2. Talen working mechanism- Core Advancement: This "one brick, one letter" design rule made TALENs' design simple, predictable, and highly successful. Scientists could target almost any DNA sequence by arranging TALE units as if spelling a word. TALENs inherited the high specificity advantage of ZFNs' "dimerization requirement for cleavage" while achieving a great leap in targeting flexibility.
- Historical Significance: TALENs were the "precision upgrade" of gene editing, transforming it from an exclusive, high-end technology into a powerful tool accessible to more laboratories, greatly accelerating research progress.
Third Generation: CRISPR – The "Gene Scissors" Revolution
- Emergence: Experienced explosive growth post-2012.
- Working Principle: The CRISPR-Cas9 system originates from a bacterial adaptive immune system. Its biggest feature is the separation of DNA recognition and cleavage functions. It requires a guide RNA (gRNA) to recognize the target sequence, while the Cas9 protein executes the cleavage. The gRNA is about 20 bases long, and its sequence is complementary to the target DNA sequence.
- Revolutionary Breakthrough: The disruptive nature of CRISPR lies in its unparalleled simplicity. To change the target, one only needs to synthesize a new short gRNA strand, which is extremely low-cost and fast. It was like moving from the era of manual machine tools to the computer numerical control (CNC) era—programming (designing gRNA) became exceptionally simple.
- Historical Significance: CRISPR technology drastically lowered the barrier to entry for gene editing, leading to its rapid adoption in countless laboratories worldwide, catalyzing a massive amount of scientific research and biotech startups—a true "revolution."
In-Depth Comparison: A Comprehensive Analysis of the Three Platforms
To visually compare the three, we first summarize with a table and then provide a detailed explanation.
| Characteristic | ZFNs | TALENs | CRISPR-Cas9 |
|---|---|---|---|
| Design Difficulty & Cost | Very High, Time-consuming, Laborious, Costly | Medium, Modular Design, Simpler than ZFNs | Very Low, Just Change gRNA, Fast & Cheap |
| Specificity & Off-Target Effects | High, but High Design Failure Rate Affects Effective Specificity | Typically Highest, Long Recognition Sequence + Dimerization Cleavage | Relatively High, but Dependent on gRNA Design, Has Off-Target Risks, Constantly Being Optimized |
| Cleavage Efficiency | Variable, Depends on Zinc Finger Assembly Quality | High and Stable, Highly Reliable by Design | Typically Very High |
| Targeting Flexibility | Limited by Availability of Zinc Finger Modules, Less Flexible | Very High, Can Target Almost Any Sequence, No PAM Restriction | Limited by PAM Sequence (e.g., SpCas9 Requires NGG) |
| Molecular Size & Delivery | Large, Challenging for In Vivo Delivery | Medium, Smaller than ZFNs, but Larger than Cas9 + gRNA | Cas9 Protein is Large, but CRISPR System is More Compact, Advantage in Delivery |
| Main Advantages | Pioneering, Long-Term Safety Data Available | High Precision, High Specificity, Targeting Flexibility | Simple, Efficient, Low-Cost, Easy to Scale |
| Main Disadvantages | Complex Design, Concentrated Patents, High Application Cost | Design Cost Higher than CRISPR, Slower Construction | Off-Target Risk, PAM Limitation, Immunogenicity Concerns |
Detailed Explanation:
- Design Difficulty & Cost: CRISPR's "Dimensionality Reduction Strike"
- ZFNs design was an "art," requiring extensive trial and error, constituting its biggest obstacle to application.
- TALENs' modular rules made its design "methodical." Through commercial kits or services, it can be accomplished relatively efficiently. However, its protein coding sequence is long, and synthesis and cloning still require time and cost.
- CRISPR's advantage is overwhelming. Enter the target sequence on a website, and you can receive synthesized gRNA within days at a very low cost. This enables large-scale, high-throughput genetic screening.
- Specificity & Off-Target Effects: TALENs' "Ace of Precision"
- This is a core advantage of TALENs. First, its recognition sequence is typically long (30-40 bp), making it unique in the vast genome. Second, the FokI nuclease must dimerize to become active, meaning two "scalpels" must be precisely positioned simultaneously to cut. This double safety mechanism greatly reduces the probability of off-target effects.
- CRISPR's gRNA recognition sequence is shorter (~20 bases) and allows some degree of mismatch, leading to a higher potential off-target risk. Although strategies like high-fidelity Cas9 variants and optimized gRNA design can mitigate this, it remains a primary concern for clinical applications.
- ZFNs themselves are highly specific, but due to the design difficulty, the successfully used ZFNs have undergone strict screening, and their actual performance specificity is high.
- Targeting Flexibility: TALENs' Freedom vs. CRISPR's "Shackles"
- TALENs can target almost any sequence in the genome, provided the sequence is accessible. This is another major advantage.
- CRISPR-Cas9's targeting ability is restricted by the PAM sequence. For example, the commonly used SpCas9 requires the target site to be followed immediately by an "NGG" sequence. This significantly limits the editable sites. Although scientists have developed Cas variants recognizing different PAMs to broaden the range, this adds complexity to the system.
- Molecular Size & Delivery: A Key Consideration for In Vivo Therapy
- For in vivo gene therapy, Adeno-Associated Virus (AAV) is often used as a delivery vector, but AAV has a limited packaging capacity.
- Smaller variants of CRISPR, like SaCas9, are more suitable for AAV packaging. However, packaging both Cas9 and gRNA into a single AAV particle remains challenging.
- The coding sequence of TALENs is smaller than ZFNs but typically larger than a single Cas9 system, posing delivery difficulties, often requiring a dual-vector strategy. This is a challenge for TALENs in in vivo applications.
Gene Editing Related Proteins
TALENs' Golden Applications: Where Does It Shine?
Despite CRISPR's prominence, TALENs, with its high specificity and target flexibility, remains irreplaceable or even superior in the following "golden scenarios":
Scenario 1: Clinical Therapies Requiring Extreme Precision
When editing human cells for disease treatment, safety is paramount. Any off-target effect could lead to cancer or other serious consequences. Therefore, in ex vivo cell therapies (e.g., CAR-T, stem cell therapies), many companies prefer using TALENs with lower off-target risks. For example, the French biotech company Cellectis' universal CAR-T therapy (UCART19) uses TALENs to knock out specific genes in T-cells to prevent rejection, with clinical trial data demonstrating the technology's safety and efficacy.
Featured CAR-T therapy target proteins
| Cat.No. | Product Name | Source | Speices | Tag |
|---|---|---|---|---|
| GPC3-2551H | Active Recombinant Human GPC3 protein, His-tagged | HEK293 | Human | His |
| EGFR-30H | Active Recombinant Human EGFR, His-tagged | HEK293 | Human | His |
| EPCAM-81H | Recombinant Human EPCAM, Fc tagged | HEK293 | Human | Fc |
| CD33-459H | Recombinant Human CD33 Molecule, Fc Chimera | HEK293 | Human | Fc |
| CD274-592H | Recombinant Human CD274 protein(Met1-Thr239), His-tagged | HEK293 | Human | His |
| CA9-256H | Recombinant Human CA9 protein, His-tagged | HEK293 | Human | His |
| TNFRSF8-642H | Active Recombinant Human TNFRSF8, Fc-His tagged | Human Cells | Human | Fc&His |
Scenario 2: "Difficult Sites" Where CRISPR is Ineffective
When the target gene lacks a suitable nearby PAM sequence, CRISPR-Cas9 is powerless. TALENs, not restricted by PAM, can freely target these "CRISPR blind spots." Additionally, for regions with extremely high GC content or particularly dense chromatin structure, TALENs can sometimes show better accessibility and editing efficiency.
Scenario 3: Strictly Regulated Fields Requiring Long-Term Safety Data
In agricultural breeding or industrial biotechnology, regulators are very cautious about approving gene-edited organisms. TALENs, due to their protein components being more "natural" (bacterial origin) and their edits being more precise and controllable (often without leaving foreign DNA), might have a clearer regulatory path in some jurisdictions and be more acceptable to the public.
Scenario 4: Constructing Stable Cell Lines or Animal Models
In drug discovery, generating stable cell lines or animal models with gene knockouts or knock-ins is essential. These models require clean editing backgrounds and minimal off-target mutations to ensure reliable experimental results. The high specificity of TALENs makes them a reliable choice for building such high-quality research tools.
How to Choose? – A Decision Guide
- If you prioritize ultimate simplicity, speed, and low cost for large-scale screening: Choose CRISPR.
- If your project demands stringent precision, involves clinical therapy or high-quality model creation, or targets sites inaccessible to CRISPR: TALENs is the more ideal and safer choice.
- ZFNs are now primarily used for specific, established platforms or research; for new projects, unless there are special patent or technical considerations, TALENs or CRISPR are generally preferred.
Conclusion: No Tool is Inherently Superior; Context Determines the Best Choice
ZFNs, TALENs, and CRISPR together form the cornerstone of modern gene editing technology. Their relationship is not simply one of "the latter replacing the former," but rather one of iteration, complementarity, and convergence. ZFNs were the pioneers, TALENs are the precision-focused mainstay, and CRISPR is the revolutionary force that democratized the technology.
As a biotech company deeply involved in the gene editing field, we fully understand that "there is no best technology, only the most suitable technology." Once you've selected your optimal technology, the next most critical decision is sourcing the high-quality protein that will drive your experiment.
At Creative BioMart, we support your research by providing the high-quality, recombinant proteins you need to bring your project to life:
- CRISPR Cas Proteins (e.g., Cas9, Cas12) for versatile targeting.
- TALEN Proteins for high-precision editing demands.
- Other Nuclease Proteins for specialized applications.
Related Products & Services
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- ADC Target Protein
- Protein Engineering Services
- Protein Expression and Purification Services
- Stable Cell Line Services
Resource
- Cas9 Enzyme: A Revolutionary Tool Ushering in a New Era of Life Sciences
- Traditional Gene Manipulation Technologies and Zinc Finger Nucleases (ZFNs)
- Cas9 Protein: Unveiling the "Molecular Scissors" of Gene Editing
- TALENs: Precision Gene Editing for Research & Therapeutics
-
Unlocking the Power of Cas9: The Future of Genetic Science
-
Unlocking CRISPR-Cas9: The Gene Editing Revolution
-
CRISPR-Cas9: Revolutionizing Genetic Science!
-
ZNFs: The Precision Tools of Gene Editing
-
Why TALENs Still Beat CRISPR for Precision Gene Editing!
-
CRISPR vs. TALEN vs. ZFNs: The Ultimate Gene Editing Battle!
References
- Isalan, M. (2011). Zinc-finger nucleases: How to play two good hands. Nature Methods, 9(1), 32-34. https://doi.org/10.1038/nmeth.1805
- Guo C, Ma X, Gao F, Guo Y. Off-target effects in CRISPR/Cas9 gene editing. Front Bioeng Biotechnol. 2023 Mar 9;11:1143157. doi: 10.3389/fbioe.2023.1143157. PMID: 36970624; PMCID: PMC10034092.
- Qiao, J., Li, W., Lin, S., Sun, W., Ma, L., & Liu, Y. (2019). Co-expression of Cas9 and single-guided RNAs in Escherichia coli streamlines production of Cas9 ribonucleoproteins. Communications Biology, 2(1), 1-6. https://doi.org/10.1038/s42003-019-0402-x
- Bhardwaj, A., & Nain, V. (2021). TALENs—An indispensable tool in the era of CRISPR: A mini review. Journal of Genetic Engineering and Biotechnology, 19(1), 1-10. https://doi.org/10.1186/s43141-021-00225-z
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

