T Cell Antigen Recognition
Related Symbol Search List
- LAX1
- CD45
- LILRA3
- LILRB1
- ILT4
- LILRB3
- LILRB4
- CD28
- CD80
- CD86
- TCF12
- LILRA4
- HLA-A
- MR1
- TAPBP
- TAPBPL
- CD3e
- CD8A
- Dectin-1
- TARP
- CD3G
- CD160
- CD1D
- CD8B1
- BST2
- CD1A
- CD1B
- CD1C
- CD1E
- CD247
- CD68
- CNPY3
- CNPY4
- HLA-G
- IFI30
- LAG3
- LAT
- PSMB10
- PSMB9
- SIT1
- TLR1
- TLR10
- TLR3
- TLR4
- TLR8
- TRAT1
- ELF1
Immunology Background
Background
About T Cell Antigen Recognition
T-cell antigen recognition is a fundamental process in adaptive immunity, enabling T cells to identify and respond to specific antigens presented by antigen-presenting cells (APCs). T cells play a critical role in immune responses, including the elimination of pathogens, tumor surveillance, and the regulation of immune reactions. The process of T-cell antigen recognition involves the interaction of T-cell receptors (TCRs) with antigenic peptides presented on major histocompatibility complex (MHC) molecules.
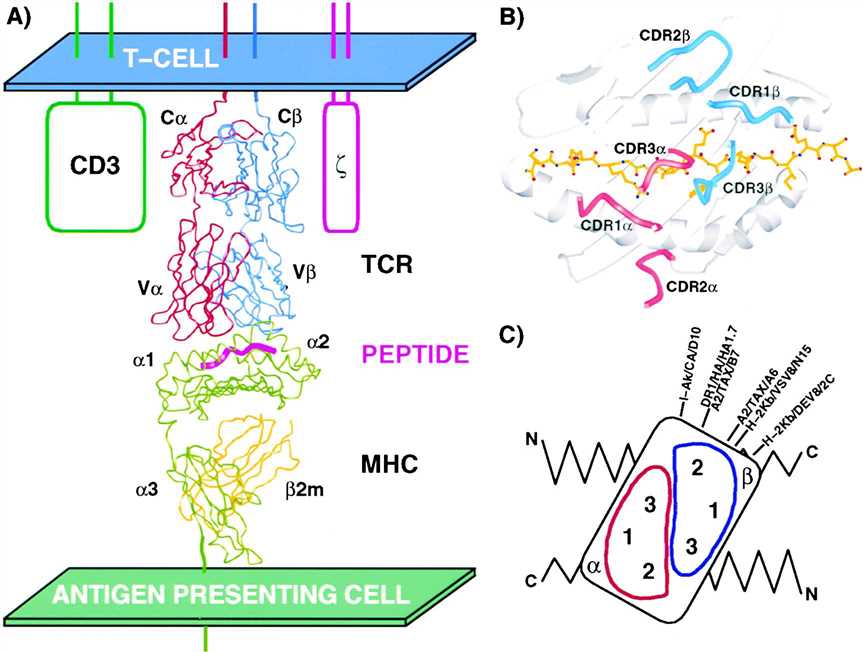 Fig.1 TCR-peptide-MHC complexes (Hennecke J, et al., 2001)
Fig.1 TCR-peptide-MHC complexes (Hennecke J, et al., 2001)Basic Principles and Mechanisms of T cell Antigen Recognition
T-cell antigen recognition is a critical process in adaptive immunity, allowing T cells to detect and respond to specific antigens presented by antigen-presenting cells (APCs). This recognition is mediated by the interaction between the TCR and the antigenic peptide-MHC complex. The principles and mechanisms underlying T-cell antigen recognition involve several key steps:
| Key steps | Basic principles and mechanisms |
|---|---|
| 1. TCR Gene Rearrangement | During T-cell development in the thymus, the genes encoding TCRs undergo a process called gene rearrangement. This process leads to the generation of a diverse repertoire of TCRs with unique antigen-binding specificities. T cells expressing TCRs that recognize self-antigens too strongly are eliminated through negative selection, ensuring tolerance to self. |
| 2. Antigen Processing and Presentation | Antigen-presenting cells, such as dendritic cells, macrophages, and B cells, capture and process foreign antigens. Intracellular pathogens are broken down into peptide fragments, while extracellular antigens are internalized and processed in endosomes. These antigenic peptides are then loaded onto MHC molecules for presentation on the cell surface. |
| 3. MHC Restriction | T-cell antigen recognition follows the principle of MHC restriction. TCRs on CD4+ helper T cells interact with antigenic peptides presented by MHC class II molecules, while TCRs on CD8+ cytotoxic T cells recognize antigenic peptides presented by MHC class I molecules. The interaction between the TCR and the peptide-MHC complex is crucial for T-cell activation. |
| 4. TCR-MHC-Peptide Interaction | The TCR recognizes the peptide-MHC complex through its variable regions, which contain complementarity-determining regions (CDRs). CDRs directly contact the antigenic peptide and the MHC molecule, forming specific interactions. The TCR-MHC-peptide interaction is highly specific, enabling T cells to distinguish between different antigens and initiate immune responses. |
| 5. Co-receptor Interactions | Co-receptors, such as CD4 and CD8, play a crucial role in T-cell antigen recognition. CD4 enhances the binding of TCRs to MHC class II molecules, while CD8 enhances the binding of TCRs to MHC class I molecules. Co-receptor engagement stabilizes the interaction between the TCR and the peptide-MHC complex, facilitating T-cell activation. |
| 6. Signal Transduction and T-cell Activation | Upon TCR engagement, intracellular signaling is initiated through the CD3 complex associated with the TCR. This signaling cascade leads to the activation of downstream signaling pathways, such as the activation of protein kinases and transcription factors. These signals ultimately result in T-cell activation, proliferation, and the initiation of effector functions. |
| 7. Co-stimulation and Co-inhibition | Co-stimulatory and co-inhibitory molecules on APCs and T cells provide additional regulatory signals during T-cell antigen recognition. Co-stimulatory molecules, such as CD28, deliver positive signals that enhance T-cell activation, while co-inhibitory molecules, such as CTLA-4 and PD-1, deliver negative signals that modulate T-cell responses and prevent excessive activation. |
By following these basic principles and mechanisms, T-cell antigen recognition ensures the specificity, regulation, and coordination of immune responses. This process allows T cells to identify and respond to antigens, leading to the elimination of pathogens, immune surveillance against tumors, and the maintenance of immune homeostasis.
Molecules Associated with T Cell Antigen Recognition
| Key molecules | Functions |
|---|---|
| T-Cell Receptors (TCRs) | TCRs are transmembrane proteins expressed on the surface of T cells. They consist of two polypeptide chains, α and β, or γ and δ, which form a heterodimer. TCRs are responsible for recognizing specific antigens presented by MHC molecules. The variable regions of TCRs (Vα and Vβ, or Vγ and Vδ) contain complementary determining regions (CDRs) that directly interact with antigenic peptides. |
| Major Histocompatibility Complex (MHC) Molecules | MHC molecules are cell surface proteins that present antigens to T cells. In humans, MHC molecules are divided into two classes: MHC class I and MHC class II. MHC class I molecules are expressed on almost all nucleated cells and present endogenous antigens to CD8+ cytotoxic T cells. MHC class II molecules are mainly expressed on APCs, such as dendritic cells, macrophages, and B cells, and present exogenous antigens to CD4+ helper T cells. The interaction between TCRs and the antigenic peptide-MHC complex is crucial for T-cell activation. |
| Co-receptors | Co-receptors are additional molecules that assist in T-cell antigen recognition and activation. They enhance the binding of TCRs to peptide-MHC complexes and provide co-stimulatory signals. The two major co-receptors are CD4 and CD8. CD4 is expressed on helper T cells and interacts with MHC class II molecules, while CD8 is expressed on cytotoxic T cells and interacts with MHC class I molecules. Co-receptor binding strengthens the interaction between TCRs and peptide-MHC complexes, facilitating T-cell activation. |
| CD3 Complex | The CD3 complex is a set of transmembrane proteins associated with the TCR on the T-cell surface. It consists of CD3γ, CD3δ, CD3ε, and ζ-chain dimers. The CD3 complex plays a crucial role in TCR signaling and T-cell activation. It provides intracellular signaling domains that initiate signal transduction upon TCR engagement, leading to downstream signaling cascades and T-cell activation. |
| CD28 and CTLA-4 | CD28 and CTLA-4 are co-stimulatory molecules expressed on T cells. CD28 provides a positive co-stimulatory signal upon binding to its ligands, CD80 (B7-1) and CD86 (B7-2), on APCs. This interaction enhances T-cell activation and proliferation. In contrast, CTLA-4 competes with CD28 for binding to CD80/CD86, but delivers an inhibitory signal, dampening T-cell activation and promoting immune tolerance. |
| PD-1 and PD-L1 | Programmed cell death protein 1 (PD-1) is an immune checkpoint receptor expressed on activated T cells. It interacts with its ligands, programmed death-ligand 1 (PD-L1) and programmed death-ligand 2 (PD-L2), which are expressed on APCs and some tumor cells. PD-1 engagement delivers inhibitory signals that regulate T-cell activation and prevent excessive immune responses. |
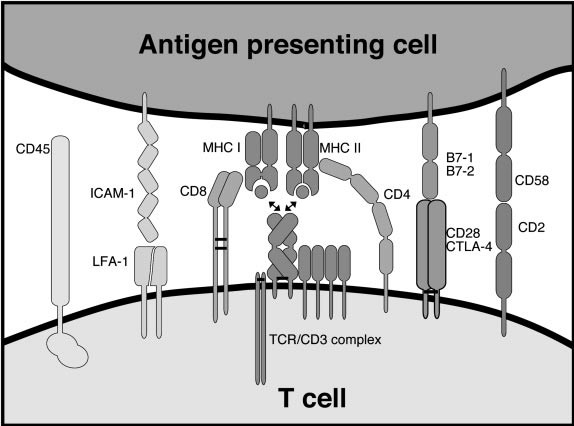 Fig.2 Cell surface molecules involved in T cell recognition. (Davis MM, et al., 2003)
Fig.2 Cell surface molecules involved in T cell recognition. (Davis MM, et al., 2003)These molecules associated with T-cell antigen recognition collectively contribute to the specificity, activation, and regulation of T-cell responses. The intricate interactions between TCRs, MHC molecules, co-receptors, and co-stimulatory/inhibitory molecules play a central role in orchestrating effective immune responses while maintaining immune tolerance and preventing autoimmunity.
T-cell Antigen Recognition in Disease Immunology
T-cell antigen recognition plays a crucial role in disease immunology, particularly in the context of infectious diseases, autoimmune disorders, and cancer. Here are some key aspects of T-cell antigen recognition in disease immunology:
Infectious Diseases
T-cell antigen recognition is vital for the immune response against infectious pathogens. During an infection, T cells recognize antigenic peptides derived from the pathogen's proteins presented on MHC molecules by infected cells or antigen-presenting cells (APCs). This recognition triggers the activation of T cells and their effector functions, such as the production of cytokines and the killing of infected cells, leading to pathogen clearance. T-cell recognition of specific antigens is essential for generating long-term immune memory, enabling a rapid and effective response upon re-exposure to the same pathogen.
Autoimmune Diseases
In autoimmune diseases, T-cell antigen recognition plays a paradoxical role. T cells are involved in recognizing self-antigens as foreign and initiating an immune response against healthy tissues, leading to tissue damage and disease. In conditions like rheumatoid arthritis, type 1 diabetes, and multiple sclerosis, autoreactive T cells recognize self-antigens presented by APCs, resulting in chronic inflammation and autoimmune pathology. Understanding and modulating T-cell antigen recognition in autoimmune diseases is crucial for developing targeted therapies that suppress or regulate the autoreactive T-cell response.
Cancer Immunology
Tumor cells can present tumor-associated antigens (TAAs) or neoantigens derived from tumor-specific mutations. T-cell recognition of these antigens plays a critical role in anti-tumor immune responses. Tumor-infiltrating T cells can recognize and eliminate cancer cells through direct cytotoxicity or by producing cytokines that stimulate other immune cells. However, tumors can evade T-cell recognition through various mechanisms, such as downregulating antigen presentation or expressing immune checkpoint molecules. Cancer immunotherapies, such as immune checkpoint inhibitors and adoptive T-cell therapies, aim to enhance T-cell antigen recognition and restore anti-tumor immune responses.
Vaccines and Immune Monitoring
T-cell antigen recognition is central to vaccine development and immune monitoring. Vaccines stimulate T-cell responses by presenting antigenic peptides in the context of MHC molecules, thereby inducing T-cell activation and memory. Understanding the specific antigens recognized by T cells during natural infections or in response to vaccines helps in designing effective vaccines. Immune monitoring techniques, such as the detection of antigen-specific T cells using MHC multimers or cytokine assays, provide insights into T-cell responses in diseases and vaccine efficacy, aiding in evaluating immune interventions and predicting clinical outcomes.
In conclusion, T-cell antigen recognition plays a critical role in disease immunology, including infectious diseases, autoimmune disorders, and cancer. Understanding the mechanisms of T-cell antigen recognition and its dysregulation in various diseases helps in developing targeted therapeutic strategies and improving immune interventions.
Case Study
Case 1: Li K, Yuan Z, Lyu J, et al. PD-1 suppresses TCR-CD8 cooperativity during T-cell antigen recognition. Nat Commun. 2021;12(1):2746.
The researchers report that PD-1 signaling modulates initial TCR antigen recognition, as evidenced by a smaller diffusion area, fewer molecular bonds, and shorter bond lifetimes for T cell interactions with peptide-major histocompatibility complex (pMHC) in the presence of PD-L1, in a manner that is dependent on SHP and the leukocyte C-terminal Src kinase.
The researchers first examined T cell spreading on coverslips functionalized with pMHC with or without PD-L1. Data suggest that PD-1 suppresses the antigen recognition process that relies on binding of the TCR-CD8 axis to the cognate pMHC, an opposite effect to LFA-1 enhancement of IS formation.
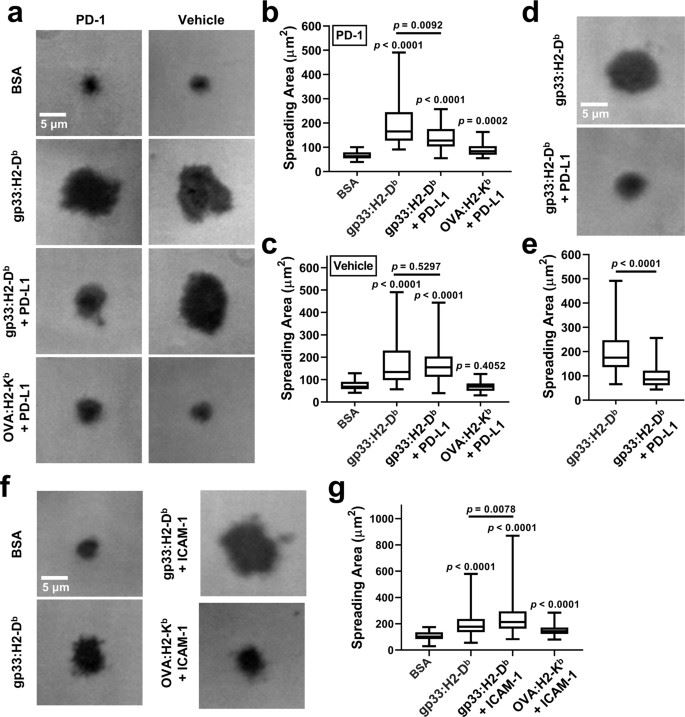 Fig.1 PD-1 inhibits T-cell spreading on surface co-presenting pMHC and PD-L1.
Fig.1 PD-1 inhibits T-cell spreading on surface co-presenting pMHC and PD-L1.Case 2: Aydin S, Pareja J, Schallenberg VM, et al. Antigen recognition detains CD8+ T cells at the blood-brain barrier and contributes to its breakdown. Nat Commun. 2023;14(1):3106.
Blood-brain barrier (BBB) rupture and immune cell infiltration into the central nervous system (CNS) are early features of multiple sclerosis (MS). Large numbers of CD8+ T cells are found in MS lesions, and antigen (Ag) presentation on the BBB has been proposed to promote CD8+ T cell entry into the CNS. Here, researchers show that brain endothelial cell processing and cross-presentation of Ag leads to effector CD8+ T cell differentiation.
To verify whether BBB endothelial cells in vivo have the potential to present antigens in an MHC class I-restricted manner, the researchers performed double immunostaining for pedicellular calyx proteins and MHC class I as vascular markers, as well as for the co-stimulatory molecules CD80, CD86, CD40, VCAM-1, and ICAM-1, which were obtained from ODC- OVA mice after day 7 of LCMV-OVA infection. OVA and WT C57BL/6 J mice. Inflamed CNS endothelial cells were confirmed to express MHC class I and key co-stimulatory molecules.
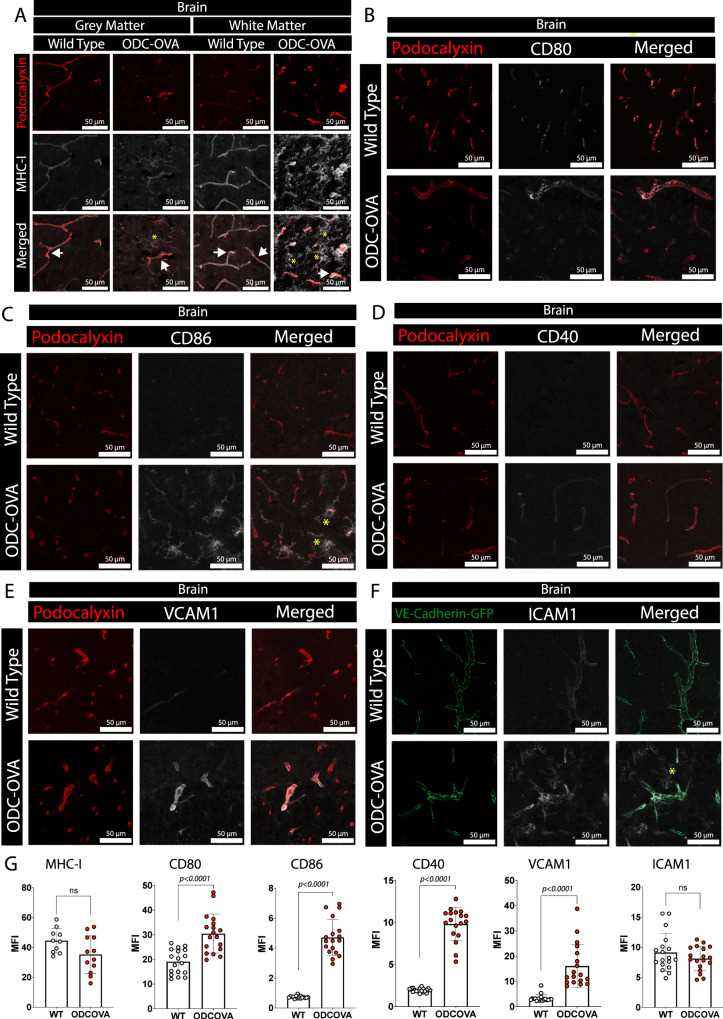 Fig.2 Inflamed brain microvascular endothelial cells express MHC class I and co-stimulatory molecules.
Fig.2 Inflamed brain microvascular endothelial cells express MHC class I and co-stimulatory molecules.References
- Hennecke J, Wiley DC. T cell receptor-MHC interactions up close. Cell. 2001;104(1):1-4.
- Davis MM, Krogsgaard M, Huppa JB, et al. Dynamics of cell surface molecules during T cell recognition. Annu Rev Biochem. 2003;72:717-742.

