CD Antigen (Regulatory T Cells)
Related Symbol Search List
- CD28
- CD38
- CD40 Ligand
- CD5
- L-selectin
- TNFRSF4
- ICAM-1
- CD4
- TLR4
- LFA-1
- CD69
- Fas
- IL7R
- CD27
- IL2RB
- IL2RA
- CD86
- ITGAE
- Neuropilin-1
- PDCD1
- RANKL
Immunology Background
Available Resources for CD Antigen (Regulatory T Cells) Research
Creative BioMart is your ultimate destination for all your research needs regarding Treg-related CD antigens. Our carefully curated selection of products and personalized services are designed to support your exploration of the intricate world of Treg-related CD antigens and their crucial role in diseases.
- Our product offerings include high-quality recombinant proteins, native proteins, protein pre-coupled magnetic beads, cell and tissue lysates, chromatography reagents, GMP proteins, assay kits, and more. Each product is meticulously tailored to meet your specific research requirements with unparalleled precision and expertise.
- Furthermore, we offer a wealth of resources on Treg-related CD antigens, providing comprehensive insights into associated pathways, protein functions, interacting proteins, relevant literature, and others.
Our Featured Products
| Cat.# | Product name | Species | Source (Host) | Tag |
|---|---|---|---|---|
| CD5-2222H | Recombinant Human CD5, His tagged | Human | Human Cell | His |
| CD27-180H | Recombinant Human CD27 protein, hFc-tagged | Human | HEK293 | hFc |
| CD86-581H | Active Recombinant Human CD86 Protein, His & Avi-tagged, Biotinylated | Human | HEK293 | His/Avi |
| IL7R-788H | Recombinant Human IL7R, Fc-His tagged | Human | Human Cell | Fc/His |
| IL2RB-783H | Active Recombinant Human IL2RB protein(Met1-Asp239) | Human | HEK293 | N/A |
| IL2RA-637H | Active Recombinant Human IL2RA, His tagged | Human | HEK293 | His |
| NRP1-157C | Recombinant Cynomolgus NRP1, Fc tagged | Cynomolgus Monkey | Human Cell | Fc/His |
| PDCD1-172H | Active Recombinant Human PDCD1, no tag | Human | HEK293 | N/A |
| ITGAE-3118H | Recombinant Human ITGAE protein, His-tagged | Human | E.coli | His |
| FAS-281H | Recombinant Human FAS, His & GST tagged | Human | Human Cell | His/GST |
| TNFRSF4-3246H | Recombinant Human TNFRSF4, Fc-His tagged | Human | Human Cell | Fc/His |
| ICAM1-656H | Recombinant Human ICAM1, Fc-His tagged | Human | Human Cell | Fc/His |
About CD Antigen (Regulatory T Cells)
Regulatory T cells (Tregs) are a specialized subset of immune cells that play a crucial role in maintaining immune tolerance and preventing excessive immune responses. They are responsible for suppressing immune reactions against self-antigens and preventing harmful immune responses to harmless substances. Tregs are derived from the same precursor cells as conventional T cells but undergo unique developmental processes that give them distinct characteristics and functions. They are primarily characterized by the expression of the transcription factor FoxP3 (forkhead box protein P3), which is considered the master regulator of Treg development and function. The main function of Tregs is to dampen immune responses and prevent immune-mediated damage. They achieve this through multiple mechanisms, including suppression of effector T cells, production of immunosuppressive molecules, cell contact-mediated suppression, and metabolic regulation.
Treg are characterized by their expression of a range of cell surface markers known as CD antigens, in particular CD4, CD25, GTLA-4, as well as FoxP3 (Forkhead Framing Protein P3) and CD127. These markers help to identify and distinguish Treg from other T cell subsets, providing important insights into the biology and function of Treg in maintaining immune tolerance and modulating immune responses.
A deeper understanding of the expression patterns and functions of CD antigens on regulatory T cells is essential for studying the underlying mechanisms of immune regulation, autoimmune diseases, transplantation, and cancer immunotherapy. Flow cytometry and other immunological techniques are commonly used to analyze the expression levels of various CD antigens on the surface of Tregs, which allows researchers and clinicians to gain a more comprehensive understanding of the role of regulatory T cells in immune homeostasis and disease, and thus better develop targeted therapies to modulate immune responses and restore immune homeostasis.
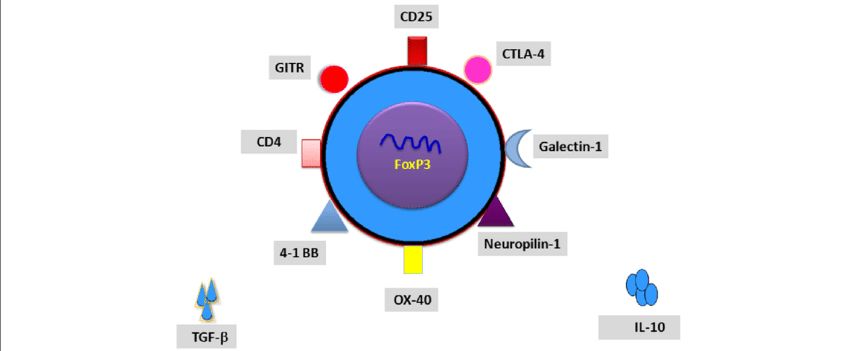 Fig.1 Schematic representation of Treg cell structure. (Rydzewska M, et al., 2018)
Fig.1 Schematic representation of Treg cell structure. (Rydzewska M, et al., 2018)
Importance of Treg CD Antigen in Diseases
- Autoimmune Diseases: In autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus, there is a breakdown of immune tolerance, leading to the immune system attacking the body's own tissues. CD antigens, particularly CD4 and FoxP3, are crucial in identifying and characterizing Tregs. Deficiencies or dysfunction of Tregs expressing these CD antigens can contribute to the development and progression of autoimmune diseases.
- Allergies and Asthma: Allergic conditions, such as allergic rhinitis and asthma, involve exaggerated immune responses to harmless substances. Tregs play a vital role in suppressing these immune responses and maintaining immune balance. CD25, a CD antigen expressed on regulatory T cells, is involved in their development and function. The altered expression or function of CD25 on regulatory T cells can contribute to the development and exacerbation of allergic and asthmatic conditions.
- Cancer Immunology: Tregs can have both beneficial and detrimental effects on cancer. On one hand, they help maintain immune homeostasis and prevent excessive immune responses against normal tissues. On the other hand, they can inhibit anti-tumor immune responses, allowing tumors to evade immune surveillance. CD antigens, such as CTLA-4 and PD-1 (PDCD1), expressed on regulatory T cells, have been targeted in cancer immunotherapy to enhance anti-tumor immune responses and improve treatment outcomes.
- Transplantation and Graft Rejection: In organ transplantation, the immune system recognizes the transplanted organ as foreign and can mount an immune response leading to graft rejection. Tregs play a crucial role in maintaining immune tolerance to transplanted organs. CD4, CD25, and FoxP3 are key CD antigens associated with regulatory T cells involved in immune tolerance. Dysregulation or altered expression of these CD antigens on regulatory T cells can contribute to graft rejection or graft-versus-host disease (GVHD) in bone marrow transplantation.
- Infectious Diseases: Tregs are involved in modulating immune responses during infections. While their role is primarily to prevent excessive immune-mediated tissue damage, their presence and function can also affect the clearance of pathogens. CD antigens, such as CD4 and CD25, are important markers for identifying and characterizing regulatory T cells in the context of infectious diseases. Understanding their expression and function can help in studying the immune response to infections and developing targeted interventions.
These examples highlight the critical role of CD antigens associated with regulatory T cells in maintaining immune balance, preventing immune-related diseases, and influencing immune responses in various health and disease contexts.
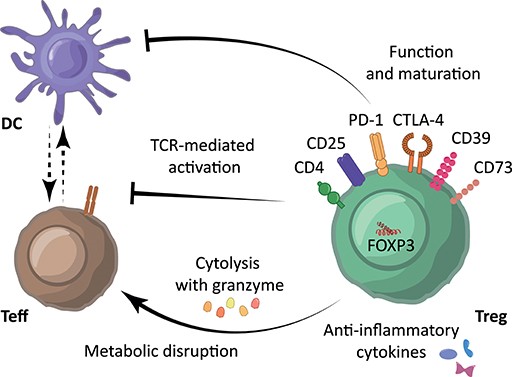 Fig.2 Immunosuppressive mechanisms underlying Treg-mediated immune suppression. (Kempkes RWM, et al., 2019)
Fig.2 Immunosuppressive mechanisms underlying Treg-mediated immune suppression. (Kempkes RWM, et al., 2019)
Treg are characterized by expression of the cell surface markers CD4+, CD25high and CD127low/−, and transcription factor FOXP3. Treg modulate the immune system using their suppressive molecules PD-1, CTLA-4, CD39, and various surface receptors through inhibition of dendritic cell (DC) function and maturation, through the secretion of anti-inflammatory cytokines such as IL-10, TGF-β and IL-35, and/or through direct inhibition of Teff via induction of cytolysis using granzyme and metabolic disruption. Moreover, Treg can reduce Teff activation by limiting TCR-ligand binding.
If you have any questions, requirements, or cooperation intentions, please feel free to contact us. We very much look forward to working with you and helping you achieve research and commercial success.
Related References
- Wegrzyn AS, Kedzierska AE, Obojski A. Identification and classification of distinct surface markers of T regulatory cells. Front Immunol. 2023;13:1055805.
- Santegoets SJ, Dijkgraaf EM, Battaglia A, et al. Monitoring regulatory T cells in clinical samples: consensus on an essential marker set and gating strategy for regulatory T cell analysis by flow cytometry. Cancer Immunol Immunother. 2015;64(10):1271-1286.
- Selck C, Dominguez-Villar M. Antigen-specific regulatory T cell therapy in autoimmune diseases and transplantation[J]. Frontiers in immunology, 2021, 12: 661875.
- Staats J. Immunophenotyping of Human Regulatory T Cells. Methods Mol Biol. 2019;2032:141-177.
- Rydzewska M, Jaromin M, Pasierowska IE, Stożek, Bossowski A. Role of the T and B lymphocytes in pathogenesis of autoimmune thyroid diseases. Thyroid Res. 2018;11:2.
- Kempkes RWM, Joosten I, Koenen HJPM, He X. Metabolic Pathways Involved in Regulatory T Cell Functionality. Front Immunol. 2019;10:2839.

