Asthma Therapeutic Targets
Related Symbol Search List
Immunology Background
Available Resources for Study of Asthma Therapeutic Targets
Creative BioMart is committed to remaining at the forefront of asthma research. Our product offerings and resources are constantly updated to provide researchers with the most current tools and information, driving progress in this crucial area of study.
- Our diverse range of products includes recombinant proteins and other essential materials that help elucidate the mechanisms of asthma. We offer a wide selection of products tailored to the unique needs of each researcher.
- Our team of experienced asthma research experts is highly knowledgeable and dedicated. They strive to deliver customized, individual solutions to meet the specific requirements of every researcher.
- Furthermore, we provide comprehensive support resources, including involved pathways, protein functions, interacting proteins, and other valuable information. These resources enable researchers to gain a comprehensive understanding of asthma, ultimately enhancing the impact of their research.
Our Featured Products
- Active Recombinant Human ITGA4 & ITGB1 Protein, His-Avi-tagged, Biotinylated
- Recombinant Human ITGA4 & ITGB7 Protein, His-Avi-tagged, Biotinylated
- Active Recombinant Human IL13
- Active Recombinant Human IL13 protein, hFc-tagged
- Recombinant Human IL13, His-tagged
- Recombinant Human IL-13 protein, His-tagged
- Recombinant Rabbit IL13 Protein
- Recombinant Zebrafish IL13 Protein
- Active Recombinant Human ICAM1 protein, His-tagged
- Recombinant Human ICAM1 protein (Met1-Glu480)
- Recombinant Human ICAM1, Fc-His tagged
- Recombinant Rhesus Monkey ICAM1 Protein
- Recombinant Bovine ICAM1 Protein
- Recombinant Mouse Icam1 Protein
- Recombinant Human Interleukin 4 Receptor
- Recombinant Human IL4R protein, His-tagged
- Recombinant Human Erythropoietin
- Active Recombinant Rat EPO, His-tagged
- Recombinant Bovine EPO Protein
- Recombinant Human PARP1, His tagged
- Recombinant Mouse Parp1 Protein, His-tagged, FITC conjugated
- Recombinant Human SELE, Fc-His tagged
- Active Recombinant Rat Sele protein(Met1-Pro494), His-tagged
- Recombinant Human SYK, His-tagged
- Recombinant Human TNFA, His-tagged
- Recombinant Zebrafish tnfa Protein
Whether you are studying Asthma pathology, drug discovery or treatment development, Creative BioMart is here to support your research. We are committed to helping you achieve your scientific goals and make meaningful contributions to the field of Asthma research. Contact us today to learn more about our products and resources.
About Asthma
Asthma is a chronic respiratory disease characterized by recurring episodes of wheezing, coughing, shortness of breath, and chest tightness. These symptoms occur due to inflammation and narrowing of the airways, leading to breathing difficulties. Asthma can vary in severity, with some individuals experiencing mild, intermittent symptoms, while others may have more frequent and severe attacks.
Asthma is a significant global health concern, affecting people of all age groups. According to the World Health Organization (WHO), as of 2021, approximately 339 million people worldwide have asthma. The prevalence of asthma varies across different countries and regions. Asthma rates tend to be higher in developed countries, urban areas, and among individuals with a family history of the condition. It is more common in children, but it can persist or develop in adulthood.
The exact causes of asthma are not fully understood, and it is likely the result of a complex interaction between genetic and environmental factors. Here are some key factors involved in the development of asthma:
- Genetic Predisposition: Asthma tends to run in families, suggesting a genetic component. Specific genes related to immune regulation and airway hyperresponsiveness have been identified, but the complete genetic basis of asthma is yet to be determined.
- Allergic Sensitization: Allergens such as pollen, dust mites, pet dander, and mold can trigger asthma symptoms in individuals with allergic asthma. When exposed to these allergens, the immune system overreacts, leading to airway inflammation and bronchoconstriction.
- Environmental Factors: Various environmental factors contribute to the development and exacerbation of asthma. These include exposure to tobacco smoke, air pollution, respiratory infections (especially in early childhood), occupational exposures to certain chemicals or irritants, and indoor allergens.
- Airway Inflammation: Inflammation plays a central role in asthma. When exposed to triggers, the airways become inflamed, leading to increased production of mucus and the recruitment of immune cells, such as eosinophils and mast cells. This inflammation causes the airway walls to swell, narrow, and become hyperresponsive.
- Airway Remodeling: Long-term inflammation and repeated episodes of asthma can lead to structural changes in the airways, known as airway remodeling. This process involves thickening of the airway walls, increased smooth muscle mass, and deposition of collagen. Airway remodeling contributes to persistent airflow limitation and reduced lung function in some individuals with asthma.
It is important to note that asthma triggers can vary between individuals, and the severity and frequency of symptoms can fluctuate over time. Effective asthma management involves identifying and avoiding triggers, using appropriate medications to control inflammation and bronchoconstriction, and developing an asthma action plan in collaboration with healthcare professionals.
Asthma Therapeutic Targets
Research on asthma treatment targets is continually evolving, with scientists exploring various cytokines, receptors, signaling pathways, and molecular mechanisms involved in the pathogenesis of asthma. Here are some prominent treatment targets currently being studied:
Interleukin-4 (IL-4) and Interleukin-13 (IL-13)
- These cytokines play a crucial role in mediating airway inflammation and promoting allergic responses in asthma.
- Targeting IL-4 and IL-13 receptors or their downstream signaling pathways is being investigated as a potential therapy. Monoclonal antibodies such as dupilumab (anti-IL-4 receptor α) and tralokinumab (anti-IL-13) have shown promising results.
Interleukin-5 (IL-5)
- IL-5 is involved in the recruitment, activation, and survival of eosinophils, which contribute to airway inflammation in asthma.
- Monoclonal antibodies targeting IL-5 or its receptor, such as mepolizumab, reslizumab, and benralizumab, have been developed to reduce eosinophilic inflammation and prevent exacerbations.
Tumor Necrosis Factor-alpha (TNF-α)
- TNF-α is a pro-inflammatory cytokine associated with asthma severity and airway remodeling.
- Clinical trials are assessing the efficacy of TNF-α inhibitors (e.g., etanercept, infliximab) in managing severe asthma and reducing inflammation.
Thymic Stromal Lymphopoietin (TSLP)
- TSLP is an epithelial cell-derived cytokine that plays a crucial role in initiating and maintaining allergic inflammation in asthma.
- Targeting TSLP or its receptors is being explored as a potential therapeutic approach to modulate the immune response in asthma.
Janus Kinase (JAK) Inhibitors
- JAK inhibitors target intracellular signaling pathways involved in inflammation and immune responses.
- Drugs like tofacitinib and baricitinib, which inhibit JAK enzymes, are being investigated for their potential use in asthma treatment.
Prostaglandin D2 (PGD2) and Prostaglandin D2 Receptor 2 (DP2)
- PGD2 and DP2 receptor (also known as CRTh2) have been implicated in allergic inflammation and bronchoconstriction in asthma.
- DP2 receptor antagonists, including fevipiprant and setipiprant, are being studied for their potential to reduce eosinophilic inflammation and improve asthma control.
Phosphodiesterase-4 (PDE4) Inhibitors
- PDE4 inhibitors target the enzyme responsible for degrading cyclic adenosine monophosphate (cAMP), which regulates airway smooth muscle relaxation and inflammation.
- These inhibitors, like roflumilast, are being investigated for their anti-inflammatory and bronchodilator effects in asthma.
These are just a few examples of the treatment targets currently being researched in asthma. Understanding the molecular mechanisms and pathogenic pathways associated with these targets helps in developing targeted therapies that can effectively control inflammation, reduce symptoms, and improve asthma management. Ongoing clinical trials and studies continue to explore and validate the efficacy and safety of these potential treatments.
New Treatments for Asthma
New treatments for asthma are being actively researched and developed, exploring innovative approaches such as biologics, gene therapy, cell therapy, and other emerging strategies. Here's an introduction to some of these novel treatments and an overview of their progress in clinical trials:
Biologics
- Biologics are targeted therapies that use monoclonal antibodies or other biologically derived molecules to interfere with specific immune pathways involved in asthma.
- Several biologics targeting various cytokines or immune cells have been approved or are under investigation for asthma treatment.
- Examples include omalizumab (anti-IgE), mepolizumab, reslizumab, and benralizumab (anti-IL-5), dupilumab (anti-IL-4 receptor α), and tralokinumab (anti-IL-13).
Gene Therapy
- Gene therapy aims to modify a patient's genetic material to address the underlying causes of asthma.
- Research is ongoing to develop gene therapies that can target specific genes involved in asthma, such as immune regulatory genes or genes related to airway inflammation.
- Gene therapy approaches are still in the early stages of development, with preclinical and early-phase clinical trials being conducted to assess safety and efficacy.
Cell Therapy
- Cell therapy involves the use of specific cells to modulate the immune response or repair damaged lung tissue in asthma.
- Mesenchymal stem cells (MSCs) have shown potential in preclinical and early clinical studies for their immunomodulatory and tissue repair properties.
- Other cell types, such as regulatory T cells (Tregs) and induced pluripotent stem cell-derived cells, are also being investigated for their potential in asthma treatment.
Small Molecule Inhibitors
- Small molecule inhibitors target specific enzymes, receptors, or signaling pathways involved in asthma pathogenesis.
- For example, inhibitors of the prostaglandin D2 receptor (DP2 receptor) and Janus kinases (JAKs) are being developed as potential treatments for asthma.
- Clinical trials are assessing the efficacy and safety of these small molecule inhibitors in asthma patients.
Precision Medicine
- Precision medicine aims to tailor treatments based on an individual's specific characteristics, such as genetic profile, biomarkers, and response to therapy.
- Advancements in genomic and proteomic profiling techniques enable the identification of molecular signatures that can guide personalized asthma treatment approaches.
- Clinical trials are underway to evaluate the effectiveness of precision medicine approaches in improving asthma outcomes.
It is important to note that while these novel treatments hold promise, many are still in the research and development stages. Clinical trials are ongoing to assess their safety, efficacy, and long-term effects. The availability of these treatments may vary depending on regulatory approvals and country-specific guidelines. Consulting with healthcare professionals and participating in clinical trials.
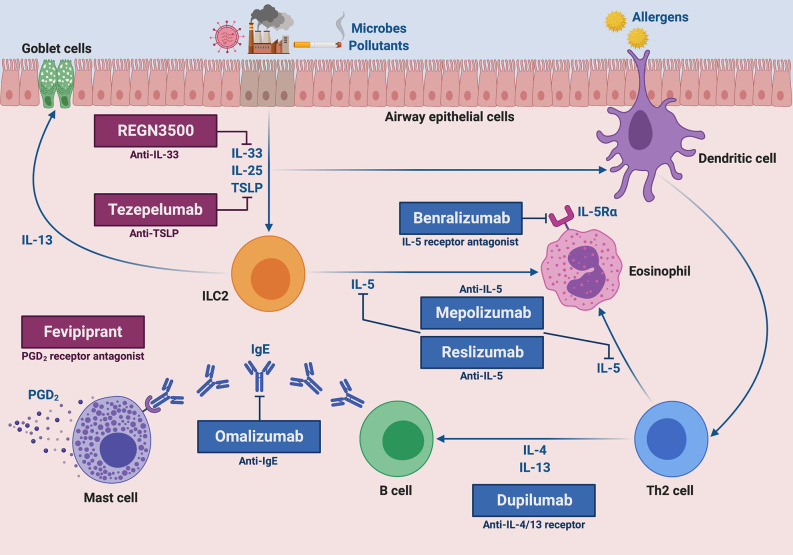 Fig.2 Molecular targets of current and future biological therapies of severe type 2 asthma. (Pelaia C, et al., 2020)
Fig.2 Molecular targets of current and future biological therapies of severe type 2 asthma. (Pelaia C, et al., 2020)
The targets of approved add-on biologic treatments (highlighted in blue color) of severe asthma include IgE (omalizumab), IL-5 (mepolizumab and reslizumab), IL-5 receptor (benralizumab), and IL-4/IL-13 receptor complex (dupilumab). Moreover, experimental drugs (highlighted in dark magenta color) such as tezepelumab, REGN3500 and fevipiprant target TSLP, IL-33 and the CRTH2 receptor of PGD2, respectively.
References:
- Pelaia C, Crimi C, Vatrella A, Tinello C, Terracciano R, Pelaia G. Molecular Targets for Biological Therapies of Severe Asthma. Front Immunol. 2020;11:603312. Published 2020 Nov 30. doi:10.3389/fimmu.2020.603312
- Chupp GL, Kaur R, Mainardi A. New Therapies for Emerging Endotypes of Asthma. Annu Rev Med. 2020;71:289-302. doi:10.1146/annurev-med-041818-020630

