Neural Stem Cell Markers
Related Symbol Search List
- NOG
- SFRP2
- ASCL1
- CTNNB1
- PRKCZ
- FZD9
- ROR2
- NEUROD1
- GFAP
- METRNL
- MSI1
- NES
- PDGFRA
- PROKR2
- VIM
- NOTCH1
- NOTCH2
- PAX3
- FABP7
- ID2
- PAX6
- SOX1
- MSX1
- ABCG2
- BMI1
- CALCR
- CDCP1
- CDH12
- CXCR4
- FABP3
- FUT4
- GATA2
- HOXB1
- MSI2
- NFE2L2
- NR2F1
- NR6A1
- OTX2
- PROM2
- RUNX1
- SLAIN1
- SMARCA4
- SOX11
- SOX21
- SOX9
- ZIC1
Immunology Background
Overview of Neural Stem Cell Markers
Neural stem cell markers are specific molecular markers used to identify and characterize neural stem cells in the nervous system. Researchers have achieved remarkable results in the identification and functional studies of neural stem cell markers. Through the analysis of cell surface markers, such as CD133, CD15, and CD24, we identified and isolated neural stem cells. We also investigated their biological properties and functions. Meanwhile, the researchers found that a variety of intracellular mechanisms as well as extracellular signaling molecules are involved in the expression and regulation process of neural stem cell markers. In addition, the researchers found that some neural stem cell object markers are abnormally expressed or functionally abnormal in neurological diseases, such as tumor formation and neurodegeneration (as in Fig. 1). Overall, advances in the study of neural stem cell markers have enriched our understanding of the properties and functions of neural stem cells. This has facilitated the developing, regenerating, and treating of developmental diseases in the nervous system by providing essential clues. Future research will continue to explore the role and regulatory mechanisms of neural stem cell markers to further promote neuroscience development and application.
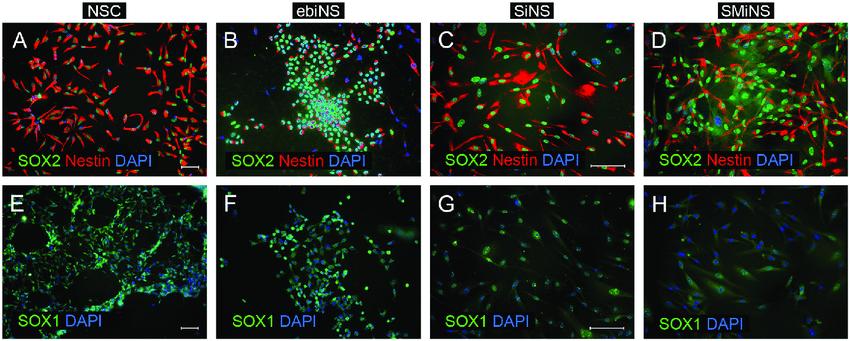 Fig.1 The expression of three neural stem cell markers SOX2, nestin, and SOX1 in NSC (A, E), ebiNSc (B, F), SiNSc-like cells (C, G), and SMiNSclike cells (D, H). (Winiecka-Klimek M, et al., 2015)
Fig.1 The expression of three neural stem cell markers SOX2, nestin, and SOX1 in NSC (A, E), ebiNSc (B, F), SiNSc-like cells (C, G), and SMiNSclike cells (D, H). (Winiecka-Klimek M, et al., 2015)Categories and Features of Neural Stem Cell Markers
Neural stem cell markers can be classified into various types, including but not limited to surface markers, transcription factors, cytoskeleton-associated proteins, and receptor/signaling proteins. These markers have different characteristics and functions in neural stem cell research.
- Surface markers
Surface markers allow the identification and isolation of neural stem cells by the expression of antigens on the cell surface. Such as ABCG2, CD15/Lewis X, CD133, CDCP1, FABP7/B-FABP, FABP8/M-FABP, PDGF R alpha, Prominin 2, ROR2, SSEA-1, SSEA-4, TRAF-4.
- Transcription factors
Transcription factors are proteins that regulate gene expression and can help maintain the self-renewal and pluripotency of neural stem cells. Such as ASCL1/Mash1, beta-Catenin, FoxD3, GATA-2, GCNF/NR6A1, HOXB1, ID2, NeuroD1, Noggin, Notch-1, Notch-2, Nrf2, Nucleostemin, Numb, Otx2, Pax3, Pax3/ Pax7, Pax6, PKC zeta, RUNX1/CBFA2, RXR α/NR2B1, SOX1, SOX2, SOX9, SOX11, SOX21, ZIC1.
- Cytoskeleton-associated proteins
Cytoskeleton-associated proteins are involved in cell structure and stability and are associated with cytoskeleton dynamics and differentiation of neural stem cells. Such as N-Cadherin and Vimentin.
- Receptors and signaling proteins
Receptors and signaling proteins are involved in the regulation of signaling pathways and cell fate decisions in neural stem cells. Such as Calcitonin R, CXCR4, FGFR2, FGFR4, Frizzled-9, Glut1, Musashi-1, Musashi-2, sFRP-2.
The study and utilization of these markers will provide a better understanding of the properties, differentiation capacity, and application potential of neural stem cells and provide important tools and information for neurological disease treatment and regenerative medicine research.
Applications and Prospects of Neural Stem Cell Markers
- Identification and isolation of neural stem cells
Neural stem cell markers can be used to identify and isolate cell populations with stem cell properties, thus enabling the purification and expansion of neural stem cells and providing high-quality samples for further research.
- Study of neural stem cell properties and Mechanisms
By studying the expression patterns and regulatory mechanisms of neural stem cell markers, we can gain insight into the self-renewal, differentiation, and proliferation processes of neural stem cells and reveal the molecular mechanisms of neural development and regeneration.
- Neurological disease treatment
The study of neural stem cell markers can help identify and select subpopulations of neural stem cells with therapeutic potential for the treatment of neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, and spinal cord injury. These markers can also be used to observe and assess neural stem cells' survival, proliferation, and differentiation after transplantation.
- Drug screening and toxicity assessment
Using neural stem cell markers, high-throughput screening platforms can be established for the discovery and evaluation of new drugs for neurological diseases, as well as for assessing compound toxicity and safety.
- The field of tissue engineering and regenerative medicine
Neural stem cell markers can guide and monitor the engineering and regeneration process of neural tissues. This provides the basis and guidance for tissue repair and regenerative medicine.
The research and application of neural stem cell markers can help advance neuroscience and provide new avenues and possibilities for treating neurological diseases, promoting neural regeneration, and improving quality of life.
Reference
- Winiecka-Klimek M, Smolarz M, Walczak MP, Zieba J, Hulas-Bigoszewska K, Kmieciak B, Piaskowski S, Rieske P, Grzela DP, Stoczynska-Fidelus E. SOX2 and SOX2-MYC Reprogramming Process of Fibroblasts to the Neural Stem Cells Compromised by Senescence. PLoS One. 2015 Nov 4;10(11): e0141688. DOI: 10.1371/journal.pone.0141688. PMID: 26535892; PMCID: PMC4633175.

