Regulatory T Cells (Tregs)
Related Symbol Search List
- CXCR3
- CXCR4
- CXCR5
- Il7
- IL7R
- CCR7
- Ifng
- TNFa
- CCR5
- CCR6
- IL12B
- IL2
- IL-4
- Il6
- IL21
- IL2RA
- IL27
- IL23A
- Tgfb1
- CCR8
- Il33
- CXCR6
- IL15
- CCL1
- CCL25
- CCL3
- CCL4
- CCR10
- CCR2
- CCR4
- CCR9
- FOXP3
- PF4
Immunology Background
Background
Regulatory T cells (Tregs) are a specialized subset of T cells (CD4+ T cells) that play a crucial role in immune regulation and maintaining immune tolerance. They are essential for preventing autoimmune diseases and excessive immune responses that could lead to tissue damage. Tregs possess unique phenotypic and functional characteristics that distinguish them from other T cell subsets.
Origin and Developmental Process
Tregs can develop from two main sources: thymus-derived Tregs (tTregs) and peripheral-induced Tregs (pTregs). tTregs arise during T cell development in the thymus, where they undergo positive and negative selection processes, leading to their differentiation into self-reactive Tregs. pTregs, on the other hand, are generated in the periphery from conventional CD4+ T cells in response to specific environmental cues, such as antigens and cytokines.
Phenotypic Characteristics
Tregs express a unique set of cell surface markers that distinguish them from other T cell subsets. Tregs are express high levels of CD25 and Forkhead box P3. They also display markers such as CTLA-4, GITR, and low levels of CD127.
Functional Characteristics
The primary function of Tregs is immune suppression and immune tolerance maintenance. They employ multiple mechanisms to suppress immune responses and prevent excessive inflammation. Tregs can directly interact with other immune cells through cell-to-cell contact, where they inhibit their activation and proliferation. Additionally, Tregs secrete immunosuppressive molecules, such as IL-10 and TGF-β, which further contribute to immune regulation.
Tregs play a vital role in preventing autoimmune diseases by suppressing self-reactive T cells. They also modulate immune responses during infections, cancer, and transplantation. Furthermore, Tregs have been shown to possess plasticity, allowing them to adapt their phenotype and function depending on the microenvironment.
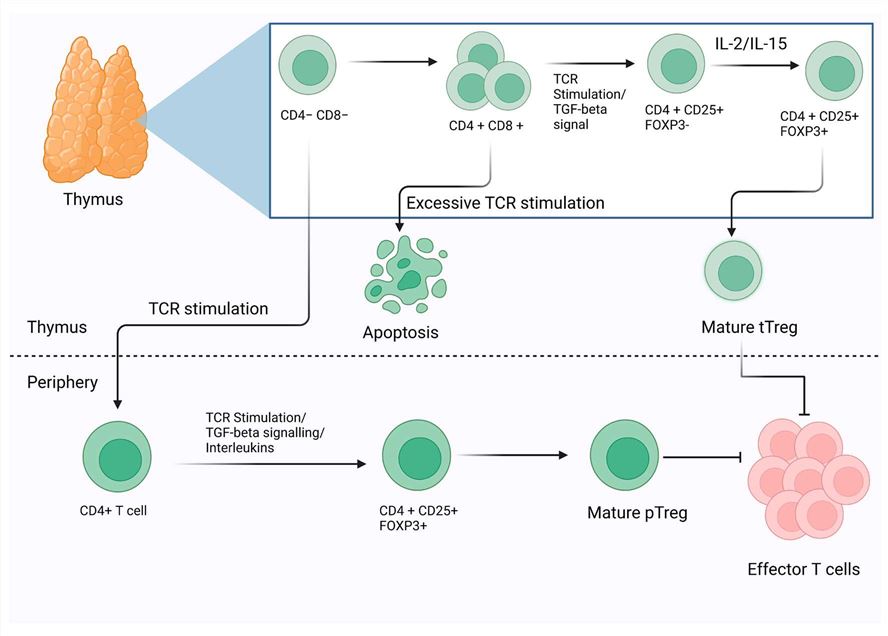 Fig.1 The schematic representation of the morphogenesis and development of Tregs. On the basis of the development and their functional markers, Tregs have been classified into two major categories thymic Tregs (tTregs) and peripheral Tregs (pTregs). Additionally, mature CD4+ Th cells can be induced into Tregs by TCR stimulation. (Dhawan M, et al., 2023)
Fig.1 The schematic representation of the morphogenesis and development of Tregs. On the basis of the development and their functional markers, Tregs have been classified into two major categories thymic Tregs (tTregs) and peripheral Tregs (pTregs). Additionally, mature CD4+ Th cells can be induced into Tregs by TCR stimulation. (Dhawan M, et al., 2023)Molecules Associated with Tregs Functions
About Molecules Associated with Tregs Functions
Tregs exert their suppressive functions through the secretion of various molecules and the expression of specific cell surface receptors. These molecules and receptors contribute to the immunosuppressive and immune-regulatory properties of Tregs. Here are some of the key molecules associated with Treg functions:
| Key molecules | Functions |
|---|---|
| Transforming Growth Factor-beta (TGF-β) | TGF-β is a cytokine produced by Tregs that plays a crucial role in immune suppression. It inhibits the activation and proliferation of effector T cells, promotes the differentiation of naive T cells into Tregs, and contributes to the maintenance of Treg stability and function. |
| Interleukin-10 (IL-10) | IL-10 is an anti-inflammatory cytokine secreted by Tregs. It has immunosuppressive effects by suppressing pro-inflammatory immune responses, inhibiting the production of inflammatory cytokines by other immune cells, and downregulating antigen presentation and co-stimulatory molecule expression. |
| Cytotoxic T-Lymphocyte-Associated Protein 4 (CTLA-4) | CTLA-4 is a cell surface receptor expressed by Tregs that plays a vital role in immune regulation. It competes with the co-stimulatory receptor CD28 for binding to CD80/CD86 on antigen-presenting cells. By doing so, CTLA-4 dampens T cell activation and enhances Treg suppressive function. |
| Programmed Cell Death Protein 1 (PD-1) | PD-1 is another cell surface receptor expressed by Tregs. It interacts with its ligands PD-L1 and PD-L2, which are expressed on various immune cells. PD-1 signaling contributes to Treg-mediated suppression by inhibiting the activation and function of effector T cells. |
| Granzyme B and Perforin | Tregs can also exert suppressive effects through the release of cytotoxic molecules such as granzyme B and perforin. These molecules can induce apoptosis in target cells, including activated T cells and antigen-presenting cells, thereby suppressing immune responses. |
| Adenosine | Tregs can produce adenosine, which acts as an immunosuppressive molecule. Adenosine inhibits the activation and function of various immune cells, including effector T cells, natural killer cells, and dendritic cells, thereby contributing to immune regulation. |
| Galectin-1 | Galectin-1 is a carbohydrate-binding protein produced by Tregs. It can induce apoptosis in activated T cells and inhibit the production of pro-inflammatory cytokines, thereby promoting immune tolerance. |
These are just a few examples of the molecules associated with Treg functions. Tregs employ a combination of mechanisms and molecules to exert their suppressive effects and maintain immune tolerance. The intricate interplay between these molecules and receptors is essential for the proper functioning of Tregs and immune homeostasis in the body.
How Treg Maintains Immune System Homeostasis by Regulating Other Immune Cells
Tregs play a crucial role in maintaining immune system homeostasis by regulating the activity of other immune cells. They employ various mechanisms to suppress and modulate immune responses, ensuring a balanced and controlled immune system. Here are some ways in which Tregs regulate other immune cells:
Suppression through Cell-to-Cell Contact
Tregs can directly interact with other immune cells, such as effector T cells, B cells, and antigen-presenting cells (APCs), through cell-to-cell contact. This contact allows Tregs to exert their suppressive effects and inhibit the activation, proliferation, and function of these immune cells. Tregs can deliver inhibitory signals and disrupt the signaling pathways necessary for immune cell activation.
Secretion of Immunomodulatory Molecules
Tregs secrete various immunosuppressive molecules that dampen immune responses and inhibit the activity of other immune cells. For example, Tregs produce cytokines like TGF-β and IL-10, which have inhibitory effects on immune cells. These cytokines can suppress the activation and proliferation of effector T cells, limit the production of pro-inflammatory cytokines, and dampen the function of APCs.
Modulation of Antigen Presentation
Tregs can influence antigen presentation by APCs. They can inhibit the maturation and activation of dendritic cells, a type of APC, and impair their ability to stimulate T cell responses. Tregs can also induce APCs to express inhibitory molecules or molecules that promote immune tolerance, thereby shaping the immune response towards tolerance rather than inflammation.
Metabolic Regulation
Tregs can modulate the metabolism of other immune cells, thereby affecting their function. Tregs have been shown to consume IL-2, a cytokine crucial for T cell activation and proliferation. By depriving effector T cells of IL-2, Tregs limit their expansion and suppress their immune response. Additionally, Tregs can compete with effector T cells for nutrients and resources, further restraining their activity.
Modulation of Co-stimulatory Molecules
Tregs express molecules such as CTLA-4 that can compete with the co-stimulatory receptor CD28 on T cells. By binding to CD80/CD86 on APCs, CTLA-4 inhibits co-stimulatory signals required for T cell activation. This competitive inhibition prevents excessive T cell activation and dampens immune responses.
Through these mechanisms, Tregs maintain immune system homeostasis by preventing excessive immune activation, dampening inflammation, and promoting immune tolerance. By regulating the activity of other immune cells, Tregs help prevent autoimmune diseases, limit tissue damage, and promote the resolution of immune responses once the threat has been eliminated.
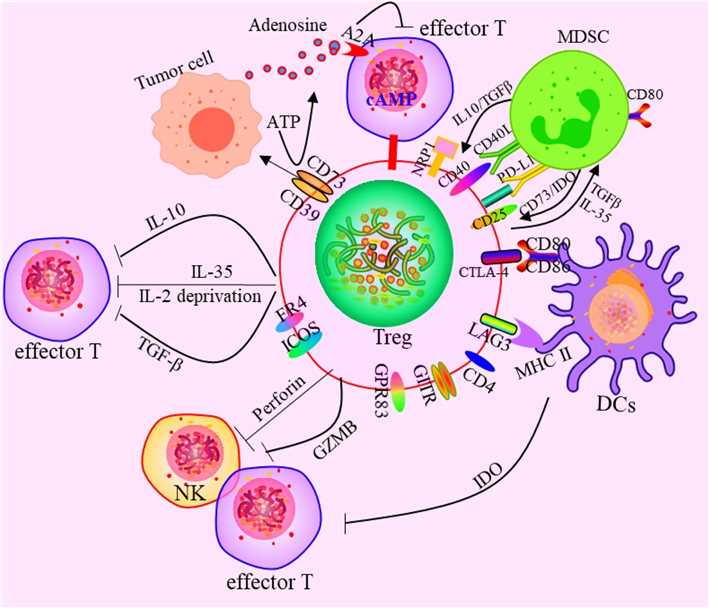 Fig.2 Effects of Tregs on the immune cells. The mechanism mainly includes four aspects: (1) Secreting inhibitory cytokines, including IL-10, TGF-β, IL-35 etc. (2) Killing effector cells by granulase and perforin. (3) Tregs affect effector cell function by interfering with cell metabolism. (4) Affect the differentiation and proliferation of Tregs by regulating DCs. (5) There exist the functional crosstalk between Tregs and MDSC. (Li C, et al., 2020)
Fig.2 Effects of Tregs on the immune cells. The mechanism mainly includes four aspects: (1) Secreting inhibitory cytokines, including IL-10, TGF-β, IL-35 etc. (2) Killing effector cells by granulase and perforin. (3) Tregs affect effector cell function by interfering with cell metabolism. (4) Affect the differentiation and proliferation of Tregs by regulating DCs. (5) There exist the functional crosstalk between Tregs and MDSC. (Li C, et al., 2020)Role of Tregs-Associated Molecules in Diseases
The molecules associated with Tregs have significant implications in various diseases. Dysregulation or alterations in the expression and function of these molecules can contribute to the development and progression of several disorders. Here are some examples of the role of Treg-associated molecules in diseases:
Autoimmune Diseases
Autoimmune diseases result from an overactive immune response against self-antigens. In conditions such as rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus, Tregs and their associated molecules play a critical role in maintaining self-tolerance and preventing autoimmune pathology. Deficiencies in Treg numbers or impaired suppressive function, along with altered expression of molecules like Foxp3, CTLA-4, and IL-10, have been associated with the development and severity of autoimmune diseases.
Cancer
Tregs play a complex role in cancer. While they are crucial for maintaining immune homeostasis, excessive Treg infiltration within the tumor microenvironment can inhibit anti-tumor immune responses and promote tumor growth. Molecules such as CTLA-4 and PD-1 expressed on Tregs contribute to immune suppression and immune evasion by tumors. Targeting these molecules with immune checkpoint inhibitors has shown promising results in cancer immunotherapy by blocking Treg-mediated immunosuppression and enhancing anti-tumor immune responses.
Infectious Diseases
During infections, Tregs help regulate immune responses to prevent excessive inflammation and tissue damage. However, in some cases, Tregs can inhibit protective immune responses, leading to chronic infections or decreased clearance of pathogens. For example, Tregs expressing molecules like CTLA-4 and PD-1 have been implicated in impairing immune responses against chronic viral infections such as HIV and hepatitis B virus.
Transplant Rejection
Tregs play a crucial role in maintaining immune tolerance to transplanted organs or tissues. In transplantation, the balance between effector T cells and Tregs is critical for graft acceptance or rejection. Treg-associated molecules like CTLA-4 and TGF-β are involved in suppressing alloreactive immune responses and promoting graft tolerance. Modulating Treg function or enhancing Treg numbers through therapies that target these molecules has the potential to improve transplant outcomes.
Allergies and Asthma
Allergic diseases, including asthma, involve dysregulated immune responses to harmless allergens. Tregs and their associated molecules, such as Foxp3 and IL-10, play a crucial role in maintaining immune tolerance and preventing excessive allergic inflammation. Deficiencies in Tregs or impaired function can contribute to allergic sensitization and the development of allergic diseases.
Understanding the role of Treg-associated molecules in diseases provides insights into the underlying immunological mechanisms and offers potential therapeutic targets. Modulating the function and activity of these molecules holds promise for the development of novel treatments aimed at restoring immune balance and ameliorating the pathology associated with various diseases.
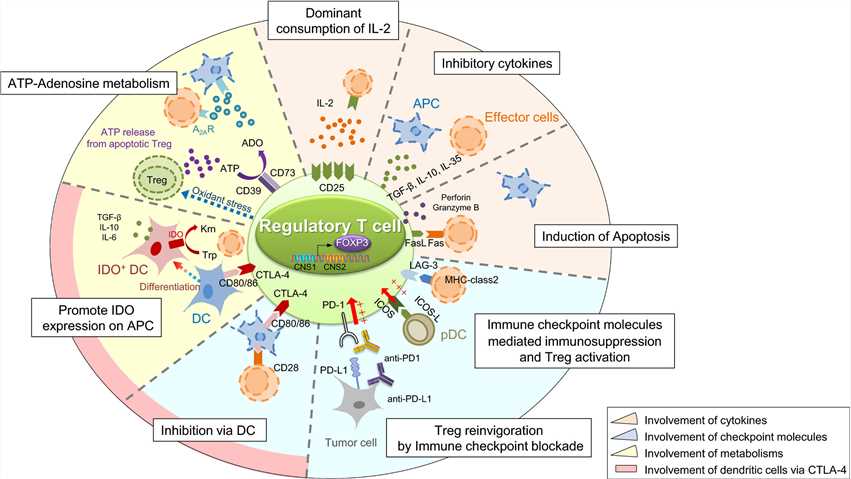 Fig.3 Suppressive mechanism of regulatory T (Treg) cells. (Ohue Y, et al., 2019)
Fig.3 Suppressive mechanism of regulatory T (Treg) cells. (Ohue Y, et al., 2019)Case Study
Case 1: Perry JA, Shallberg L, Clark JT, et al. PD-L1-PD-1 interactions limit effector regulatory T cell populations at homeostasis and during infection. Nat Immunol. 2022;23(5):743-756.
While much is known about the factors that promote the development of diverse Treg cell responses, less is known about the pathways that constrain Treg cell activities. The studies presented here reveal that at homeostasis there is a population of effector Treg cells that express PD-1, and that blockade of PD-L1 or loss of PD-1 results in increased Treg cell activity. In response to infection with the parasite T. gondii, the early production of IFN-γ results in widespread upregulation of PD-L1. Moreover, blockade of PD-L1, whole body deletion of PD-1, or lineage-specific deletion of PD-1 in Foxp3+ cells prevented the loss of the effector Treg cells but resulted in reduced pathogen specific CD4+ T cell responses during infection. Thus, at homeostasis basal PD-L1 expression constrains and tunes the pool of Treg cells, but during infection the upregulation of PD-L1 provides a mechanism to contract the Treg-cell population required to maximize the development of pathogen specific CD4+ T cell responses.
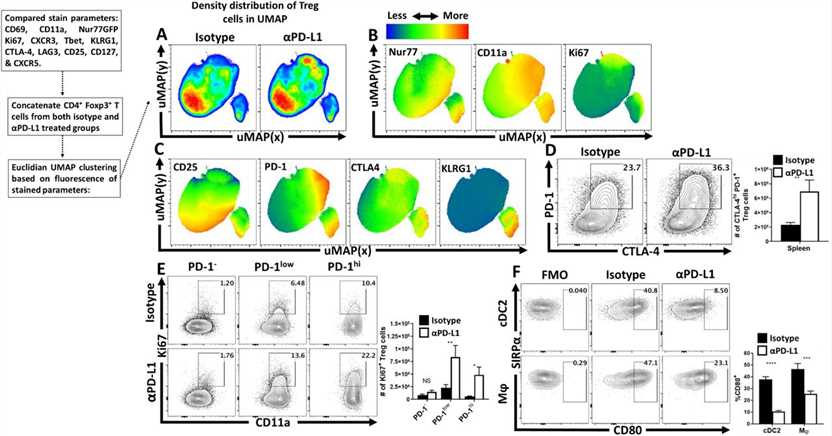 Fig.1 Anti-PD-L1 blockade results in increased eTreg cell activation and proliferation in naïve hosts.
Fig.1 Anti-PD-L1 blockade results in increased eTreg cell activation and proliferation in naïve hosts.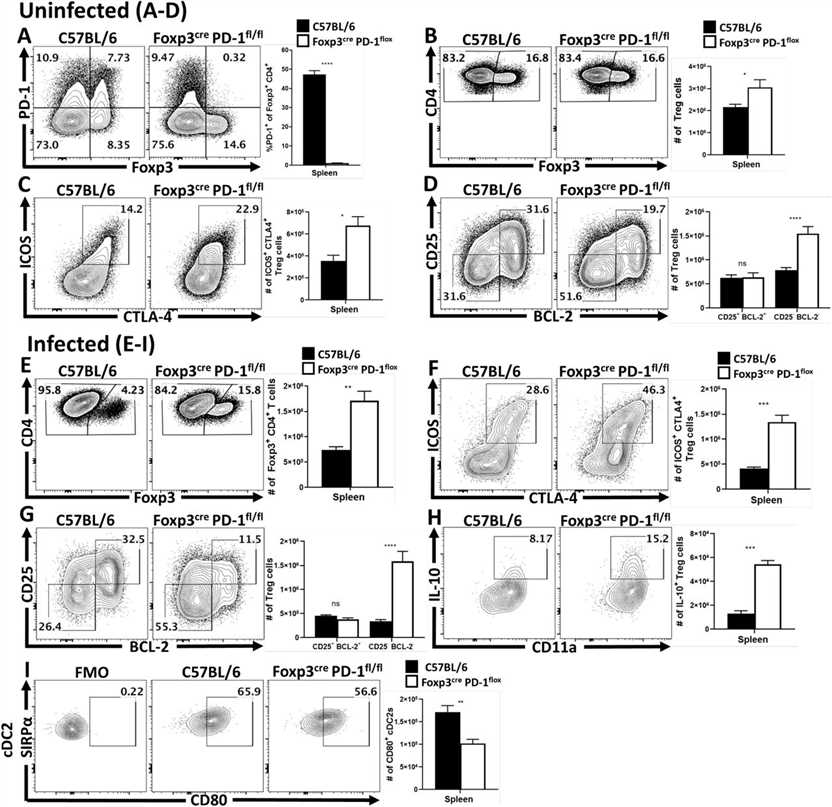 Fig.2 Treg cell-specific deletion of PD-1 enhances eTreg cell populations at homeostasis and prevents Treg cell depletion during infection.
Fig.2 Treg cell-specific deletion of PD-1 enhances eTreg cell populations at homeostasis and prevents Treg cell depletion during infection.Case 2: Zappasodi R, Serganova I, Cohen I J, et al. CTLA-4 blockade drives loss of Treg stability in glycolysis-low tumours[J]. Nature, 2021, 591(7851): 652-658.
In this paper, researchers investigated the effect of CTLA-4 blockade on the metabolic capacity of T cells within tumors in relation to the glycolytic capacity of tumor cells. The researchers found that CTLA-4 blockade promoted metabolic fitness and immune cell infiltration, especially in tumors with low glycolysis.
The researchers found that CTLA-4 blockade induced a loss of stability of glucose-dependent Treg function.
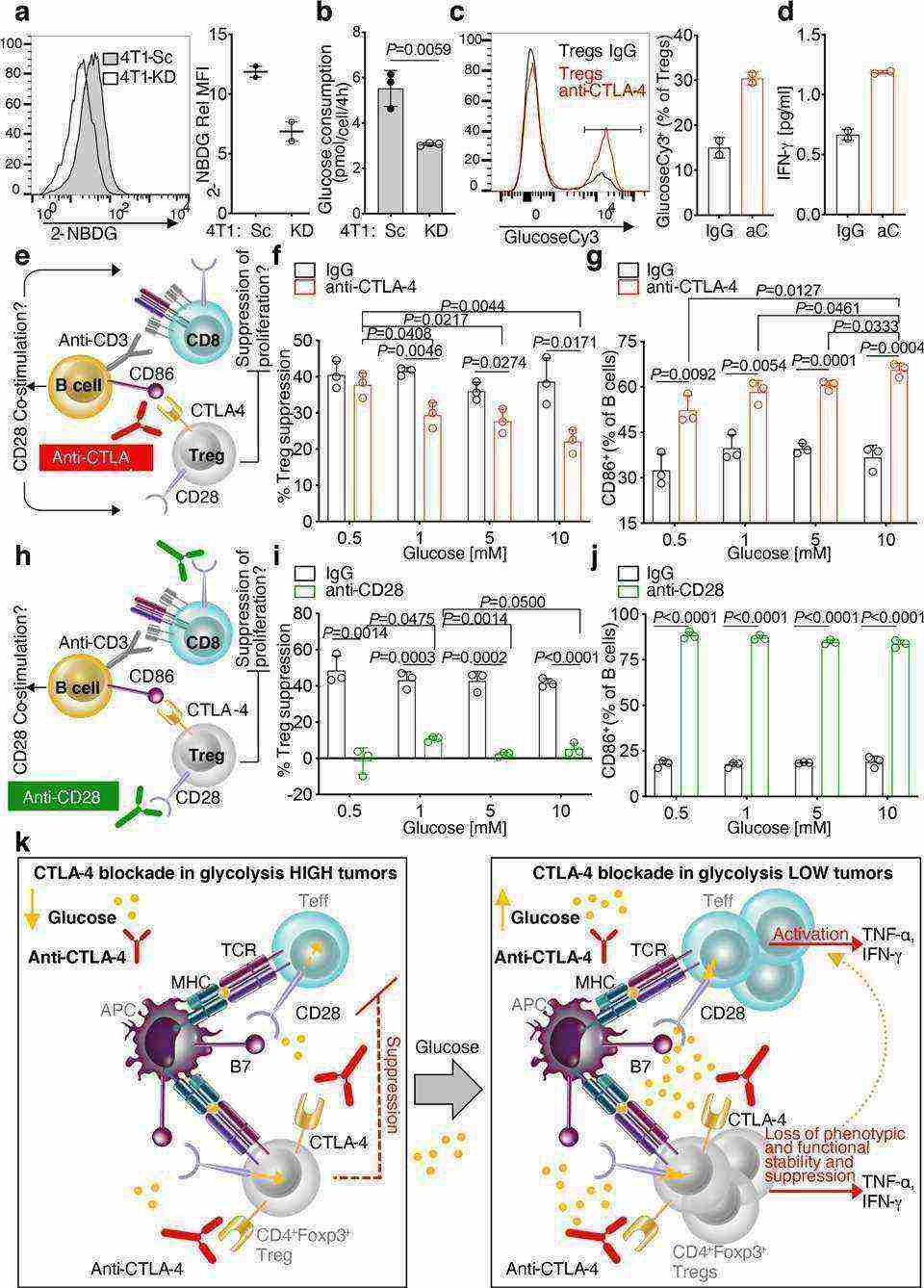 Fig.3 Glucose-dependent loss of Treg functional stability induced by CTLA-4 blockade.
Fig.3 Glucose-dependent loss of Treg functional stability induced by CTLA-4 blockade.References
- Dhawan M, Rabaan AA, Alwarthan S, Alhajri M, Halwani MA, Alshengeti A, Najim MA, Alwashmi ASS, Alshehri AA, Alshamrani SA, et al. Regulatory T Cells (Tregs) and COVID-19: Unveiling the Mechanisms, and Therapeutic Potentialities with a Special Focus on Long COVID. Vaccines. 2023; 11(3):699.
- Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 2020;19(1):116.
- Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why?. Semin Cancer Biol. 2012;22(4):327-334.
- Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target?. Cancer Sci. 2019;110(7):2080-2089.

