T Cell Migration
Related Symbol Search List
- CD81
- CD45
- LAIR1
- CD226
- SPN
- CD147
- CXCR3
- CXCR4
- CXCR5
- IGSF8
- ITGA4
- ITGAM
- ITGAX
- LTBR
- L1CAM
- LTB4R
- LTB4R2
- S1PR1
- L-selectin
- Selplg
- CCR7
- CCR5
- CCR6
- CD83
- CD10
- CCR1
- CCR3
- CCR8
- CXCR6
- ITGAE
- CCR2
- CCR4
- CCR9
- ALCAM
- CD2AP
- CD6
- CD7
- CXCR1
- CXCR2
- CYTH1
- ICAM-1
- ICAM-2
- CD31
- GPR44
- PTGDR2
- RAP1A
- SIRPG
- SLAMF9
- TLN1
- VNN1
Immunology Background
Background
T-cell migration refers to the movement of T cells from one location to another within the body. It is a dynamic process that plays a critical role in immune surveillance, immune responses, and the regulation of immune-mediated diseases. T cells are a type of white blood cell that orchestrates immune responses by recognizing and eliminating pathogens and abnormal cells. T-cell migration is essential for T cells to reach the sites where their immune functions are needed. Here's an introduction to T-cell migration:
Chemotaxis and Chemoattractants
T-cell migration is guided by chemotaxis, the movement of cells in response to chemical signals. Chemotactic signals, known as chemoattractants, direct T cells towards specific tissues or microenvironments. Chemoattractants are small molecules, such as chemokines, that bind to receptors on the surface of T cells and trigger intracellular signaling pathways leading to migration.
Adhesion Molecules and Extravasation
T-cell migration involves interactions between adhesion molecules on T cells and endothelial cells lining blood vessels. These interactions are crucial for T cells to exit the bloodstream and enter tissues, a process known as extravasation. Adhesion molecules, such as integrins and selectins, mediate the tethering, rolling, and firm adhesion of T cells to endothelial cells, allowing them to transmigrate across the blood vessel wall.
Lymphocyte Homing and Trafficking
T cells exhibit tissue-specific homing and trafficking patterns. Different tissues express unique combinations of chemokines and adhesion molecules that guide T cells to specific locations. For example, skin-homing T cells express cutaneous lymphocyte-associated antigen (CLA) and CCR4 chemokine receptor, enabling them to migrate to the skin. Gut-homing T cells express integrin α4β7 and CCR9, which facilitate their migration to the intestinal mucosa.
Lymphoid Organ Migration
T cells migrate between lymphoid organs, such as lymph nodes and the spleen, to encounter antigen-presenting cells (APCs) and initiate immune responses. Naïve T cells continuously circulate through lymphoid organs, scanning for antigens presented by APCs. This migration is driven by chemokine gradients and adhesion molecules present in the lymphoid tissue microenvironment.
Inflammatory Response and Tissue Migration
During an immune response, T cells are recruited to sites of infection, inflammation, or tissue injury. Inflamed tissues release chemokines and cytokines that attract T cells to the site. In response to these signals, T cells extravasate from blood vessels, migrate through the tissue, and interact with other immune cells to mount an appropriate immune response.
Understanding the mechanisms and regulation of T-cell migration is crucial for various aspects of immunology, including vaccine development, immune therapies, and the treatment of immune-related diseases. Researchers study the signaling pathways, adhesion molecules, and chemotactic gradients involved in T-cell migration to gain insights into immune responses and develop strategies to modulate T-cell behavior in different contexts.
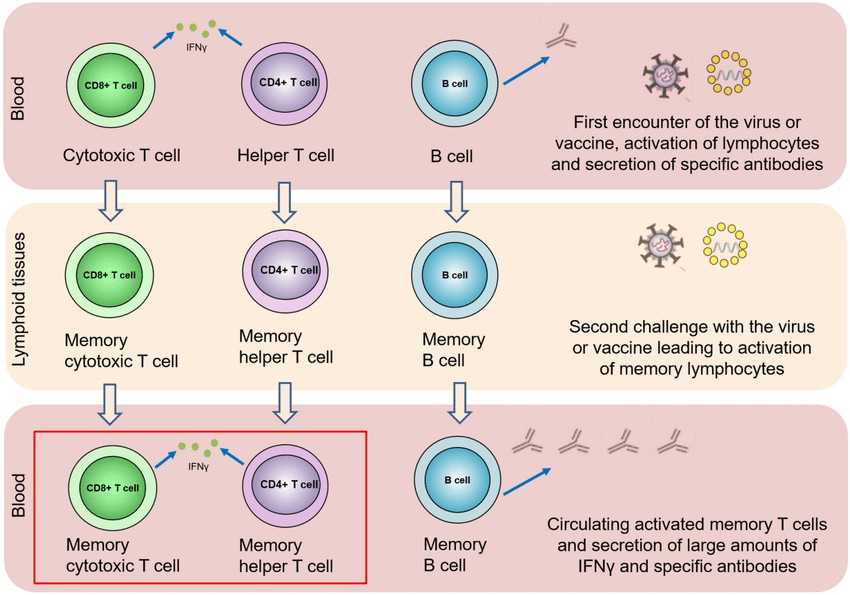 Fig.1 Localization and migration of T cells in the course of the immune response. (Kurteva E, et al., 2022)
Fig.1 Localization and migration of T cells in the course of the immune response. (Kurteva E, et al., 2022)Molecules Associated with T Cell Migration
Several molecules are associated with T cell migration. These molecules play critical roles in guiding T cells to specific tissues and mediating their interactions with endothelial cells during extravasation. Here are some key molecules involved in T cell migration:
| Key molecules type | Functions |
|---|---|
| Chemokines | Chemokines are small signaling proteins that function as chemoattractants, guiding T cells to specific tissues or microenvironments. Different tissues secrete distinct chemokines that bind to chemokine receptors on T cells, initiating intracellular signaling pathways that promote migration. Examples of chemokines involved in T cell migration include CCL19, CCL21, CXCL9, CXCL10, and CXCL12. |
| Chemokine Receptors | Chemokine receptors are expressed on the surface of T cells and bind to specific chemokines, initiating migration. The expression of chemokine receptors determines the tissue tropism of T cells. For example, CCR7 is expressed on T cells and interacts with CCL19 and CCL21, guiding T cells to lymphoid tissues. CXCR3 interacts with CXCL9 and CXCL10, attracting T cells to sites of inflammation. |
| Integrins | Integrins are cell adhesion molecules that play a crucial role in T cell migration and extravasation. Integrins on T cells, such as α4β1 (VLA-4) and αLβ2 (LFA-1), interact with adhesion molecules expressed on endothelial cells, facilitating T cell adhesion and transmigration across the blood vessel wall. |
| Selectins | Selectins are adhesion molecules expressed on endothelial cells that mediate the initial tethering and rolling of T cells along blood vessels. P-selectin and E-selectin interact with their ligands, including P-selectin glycoprotein ligand-1 (PSGL-1) and E-selectin ligand-1 (ESL-1), on T cells, allowing them to interact with the endothelium and slow down to facilitate extravasation. |
| Sphingosine-1-phosphate (S1P) and Sphingosine-1-phosphate Receptors (S1PRs) | S1P is a bioactive lipid that regulates T cell migration. It gradients of S1P guide T cells from lymphoid tissues into circulation. S1P receptors, such as S1P1, are expressed on T cells and mediate their response to S1P, influencing their migration and tissue egress. |
| Adhesion Molecules | Various adhesion molecules are involved in T cell migration and extravasation. These include vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and mucosal addressin cell adhesion molecule-1 (MAdCAM-1), which are expressed on endothelial cells and interact with integrins on T cells to facilitate firm adhesion and transmigration. |
Understanding the molecular mechanisms underlying T cell migration is crucial for developing therapies targeting immune responses and immune-related diseases. By manipulating the interactions between these molecules, researchers can modulate T cell migration, potentially improving immune surveillance, immune responses, and the treatment of immune-mediated disorders.
Role of T Cell Migration in Diseases
T cell migration and the molecules associated with it play essential roles in various diseases. Here are some examples of how T cell migration and related molecules contribute to different disease contexts:
Autoimmune Diseases
In autoimmune diseases, T cells mistakenly recognize self-antigens and mount immune responses against healthy tissues. T cell migration is crucial for autoimmune pathogenesis as T cells infiltrate target tissues, leading to tissue damage and inflammation. For instance, in multiple sclerosis, autoreactive T cells migrate across the blood-brain barrier and infiltrate the central nervous system, contributing to neuroinflammation and demyelination. Chemokines, adhesion molecules, and integrins orchestrate the migration of autoreactive T cells to target tissues in autoimmune diseases.
Inflammatory Bowel Disease (IBD)
Inflammatory bowel disease, including Crohn's disease and ulcerative colitis, is characterized by chronic inflammation in the gastrointestinal tract. T cells, particularly Th1 and Th17 cells, play a significant role in IBD pathogenesis. Chemokine receptors, such as CCR9 and integrin α4β7, guide T cells to the gut mucosa, contributing to the chronic inflammation observed in IBD. Targeting these molecules has been explored as a therapeutic strategy for IBD.
Cancer
T cell migration is critical for effective antitumor immune responses. Tumor-infiltrating lymphocytes (TILs), including cytotoxic CD8+ T cells, are crucial for recognizing and eliminating cancer cells. Chemokines produced by tumors and the tumor microenvironment guide T cells to the tumor site. However, tumors can also exploit immune evasion mechanisms to hinder T cell migration or create an immunosuppressive environment. Enhancing T cell migration and overcoming immunosuppression are important strategies in cancer immunotherapy.
Infectious Diseases
T cells play a key role in immune responses against pathogens. During infection, T cells migrate to infected tissues to eliminate pathogens. Chemokines and chemokine receptors guide T cells to the site of infection, while adhesion molecules facilitate T cell extravasation. For example, in HIV infection, CD8+ T cells migrate to lymphoid tissues and sites of active viral replication. Understanding T cell migration in infectious diseases aids in developing vaccines and therapeutics to enhance immune responses.
Transplant Rejection
T cell migration is involved in transplant rejection, where the immune system recognizes the transplanted organ as foreign and mounts an immune response against it. Alloreactive T cells migrate to the transplanted organ, causing tissue damage and rejection. Chemokines and adhesion molecules mediate the migration of alloreactive T cells, and targeting these molecules can help modulate transplant rejection.
Understanding the role of T cell migration and related molecules in disease contexts is crucial for developing targeted therapies and interventions. Modulating T cell migration can potentially enhance immune responses against pathogens, suppress autoimmune reactions, and improve the efficacy of immunotherapies in cancer and transplantation.
Case Study
Case 1: Westermann J, Söllner S, Ehlers E M, et al. Analyzing the migration of labeled T cells in vivo: an essential approach with challenging features. Laboratory investigation, 2003, 83(4): 459-469.
The interpretation of the results obtained in experiments that investigate the migration of T cells in vivo is influenced by many factors. Radioactively labeled naïve and effector T cells are injected intravenously and analyzed 1 day later. If the data are presented as "percentage of recovered radioactivity," then naïve T cells prevail in lymph nodes and spleen, whereas in all other organs, effector T cells are more numerous. If the same data are presented as "percentage of injected radioactivity," then naïve T cells are predominant in 7 of 11 organs, including many nonlymphoid organs. Furthermore, naïve T cells migrate into the bone marrow in higher numbers than effector T cells.
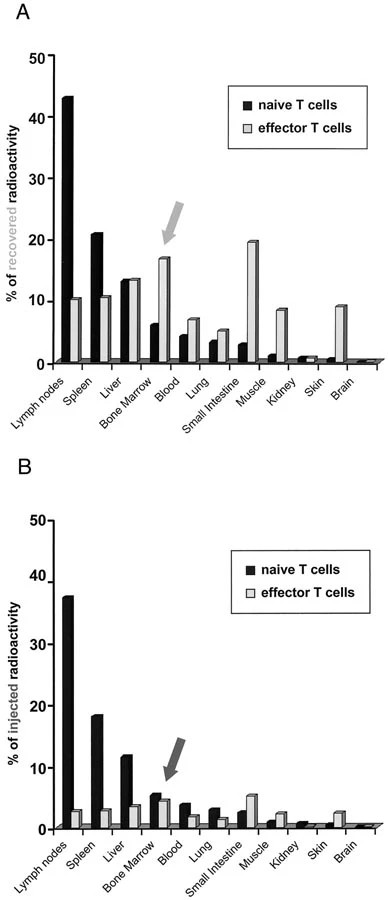 Fig.1 Migration of naïve and effector T cells in vivo: Impact of data presentation.
Fig.1 Migration of naïve and effector T cells in vivo: Impact of data presentation.Case 2: de Haan L, Suijker J, van Roey R, Berges N, Petrova E, Queiroz K, Strijker W, Olivier T, Poeschke O, Garg S, et al. A Microfluidic 3D Endothelium-on-a-Chip Model to Study Transendothelial Migration of T Cells in Health and Disease. International Journal of Molecular Sciences. 2021; 22(15):8234.
To investigate whether T cells in our co-culture model could be triggered to migrate by the addition of chemo-attractants, the chemokine CXCL12 was added to the bottom perfusion channels at the start of the co-culture. CXCL12 is a ligand for CXCR4, expressed by leukocytes, and extensively used in immunology research. The addition of CXCL12 did not affect vascular permeability, indicated by the comparable relative Papp values. The quantification of T cell numbers showed that CXCL12 significantly increased the number of unstimulated T cells residing in the vessel at 24- and 48-h timepoints. Interestingly, the number of stimulated T cells migrating into the ECM increased greatly in response to the addition of CXCL12, while the migration of unstimulated T cells was hardly affected. Although the addition of CXCL12 undeniably affects the migration of stimulated T cells, the extent to which it does varies greatly between donors. Immunofluorescent staining for CD45 verified the presence of an increased number of stimulated T cells in the ECM compartment in response to CXCL12.
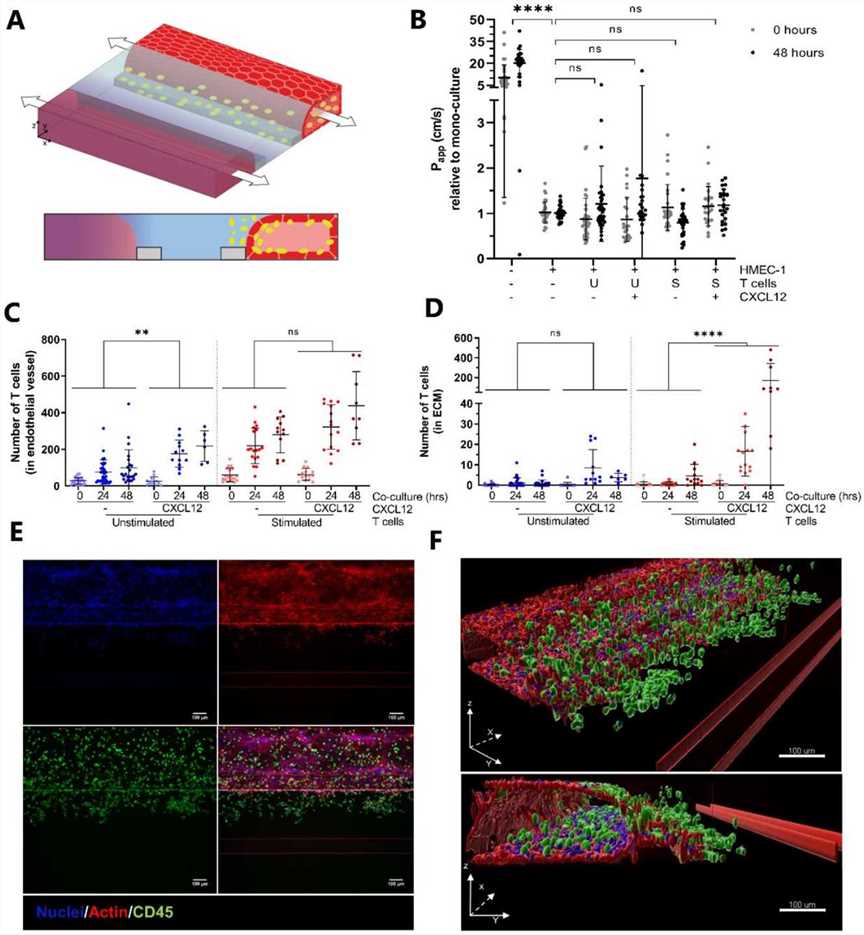 Fig.2 Addition of the chemokine CXCL12 increases T cell migration.
Fig.2 Addition of the chemokine CXCL12 increases T cell migration.References
- Kurteva E, Vasilev G, Tumangelova-Yuzeir K, et al. Interferon-gamma release assays outcomes in healthy subjects following BNT162b2 mRNA COVID-19 vaccination. Rheumatology International, 2022, 42(3): 449-456.
- Krummel MF, Bartumeus F, Gérard A. T cell migration, search strategies and mechanisms. Nat Rev Immunol. 2016;16(3):193-201.
- Fowell, Deborah J., and Minsoo Kim. The spatio-temporal control of effector T cell migration. Nature Reviews Immunology 2021, 21(9): 582-596.

