T Cell Cytokine Signaling
Related Symbol Search List
- CRLF2
- IL2RG
- IL7R
- Ifng
- TNFB
- TNFa
- Csf2
- EOMES
- IL-10
- IL12B
- IL-13
- IL17A
- IL18R1
- IL2
- IL23R
- IL-4
- Il5
- Il6
- TBX21
- IFNGR1
- IL2RA
- IL4R
- IL5RA
- Il6ra
- IL6ST
- NFATC1
- IL12RB1
- IL12RB2
- IL18
- IL1B
- IL1R1
- IL23A
- Tgfb1
- TGFBR1
- TGFBR2
- Il33
- ZAP70
- CSF2RA
- CSF2RB
- IFNGR2
- IL17F
- Il1a
- IL24
- IL3
- IL32
- NFATC2
- NFATC3
- TARM1
- TGFBR3
- TNFRSF1A
- TNFRSF1B
- RANKL
Immunology Background
Background
T-cell cytokine signaling refers to the communication process between T cells, a type of white blood cell, through the release and reception of signaling molecules called cytokines. Cytokines are small proteins that play essential roles in regulating immune responses by facilitating communication between different immune cells.
T cells are a vital component of the adaptive immune system and are responsible for coordinating and executing immune responses against pathogens, tumors, and other foreign substances. When T cells recognize specific antigens presented by antigen-presenting cells (APCs), they become activated and secrete various cytokines.
Upon activation, T cells can differentiate into distinct subsets, including helper T cells (Th cells) and cytotoxic T cells (Tc cells). Each subset produces a unique set of cytokines that modulate immune responses in different ways.
- Helper T cells, such as Th1, Th2, Th17, and regulatory T cells (Tregs), release cytokines that influence the behavior of other immune cells. For example, Th1 cells produce interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), which promote cellular immunity and activation of macrophages. Th2 cells secrete interleukins (IL-4, IL-5, IL-13), which drive humoral immune responses and help in the clearance of extracellular pathogens. Th17 cells produce IL-17 and IL-22, which are involved in combating fungal and bacterial infections. Tregs release transforming growth factor-beta (TGF-β) and IL-10, contributing to immune tolerance and regulation.
- Cytotoxic T cells, also known as CD8+ T cells or Tc cells, play a crucial role in eliminating infected or abnormal cells. Upon activation, they produce cytokines like IFN-γ and TNF-α, which enhance their cytotoxic capabilities and recruit other immune cells to the site of infection or tumor.
T-cell cytokine signaling is a complex network of interactions that regulates the intensity, duration, and specificity of immune responses. Cytokines act in an autocrine (acting on the same cell that secretes them), paracrine (acting on nearby cells), or endocrine (acting on distant cells) manner. They bind to specific receptors on target cells, triggering a cascade of intracellular signaling events that modulate gene expression, cell differentiation, proliferation, and immune effector functions.
The dysregulation of T-cell cytokine signaling can have profound implications for immune-mediated diseases. Excessive or prolonged cytokine signaling can lead to chronic inflammation, autoimmune disorders, or immune deficiencies. Conversely, impaired cytokine signaling can result in reduced immune responses and increased susceptibility to infections.
Understanding T-cell cytokine signaling is crucial for elucidating the mechanisms of immune regulation, developing targeted immunotherapies, and designing vaccines. Manipulating cytokine signaling pathways has the potential to modulate immune responses, promote protective immunity, and treat various immune-related disorders.
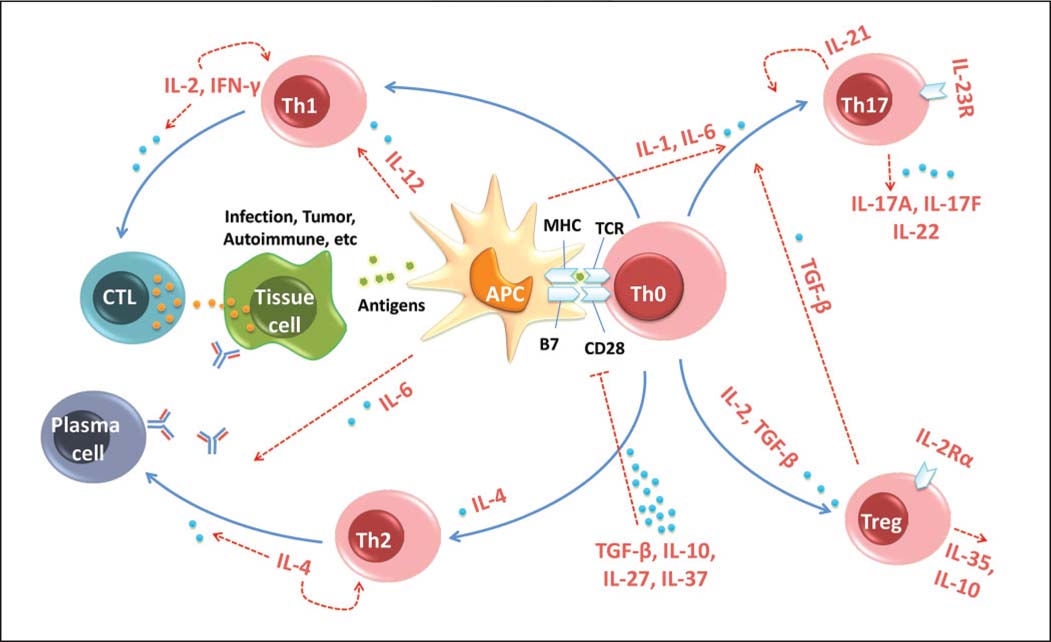 Fig.1 Cytokines regulate immune tolerance by affecting T cell activation and differentiation. (Wu J, et al., 2014)
Fig.1 Cytokines regulate immune tolerance by affecting T cell activation and differentiation. (Wu J, et al., 2014)Molecules Associated with T Cell Cytokine Signaling
Several molecules are associated with T cell cytokine signaling. These molecules play critical roles in transmitting signals and regulating the production and response to cytokines in T cells. Here are some key molecules involved in T cell cytokine signaling:
| Key molecule types | Functions |
|---|---|
| Cytokines | Cytokines themselves are the primary signaling molecules in T cell cytokine signaling. They are produced and secreted by T cells upon activation and act as messengers to communicate with other immune cells. Examples of cytokines produced by T cells include interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), interleukins (IL-2, IL-4, IL-6, IL-10, IL-17, etc.), transforming growth factor-beta (TGF-β), and many others. Each cytokine has specific functions and can elicit distinct immune responses. |
| Cytokine Receptors | Cytokine receptors are transmembrane proteins expressed on the surface of T cells. They bind to specific cytokines, allowing the transmission of signals into the cell. Cytokine receptors are typically composed of multiple subunits, including ligand-binding subunits and signaling subunits. The binding of cytokines to their receptors triggers intracellular signaling cascades, leading to various cellular responses. Examples of cytokine receptors include the IL-2 receptor (IL-2R), IL-4 receptor (IL-4R), IL-6 receptor (IL-6R), and TNF receptor (TNFR) family members. |
| JAKs and STATs | Janus kinases (JAKs) and signal transducers and activators of transcription (STATs) are key molecules involved in cytokine signaling pathways. Upon cytokine receptor activation, JAKs are recruited to the receptor complex and phosphorylate specific tyrosine residues on the receptor and STATs. Phosphorylated STATs then form dimers, translocate to the nucleus, and regulate gene expression. Different cytokines can activate different combinations of JAKs and STATs, leading to the induction of specific genes and cytokine responses. |
| Suppressor of Cytokine Signaling (SOCS) Proteins | Suppressor of cytokine signaling (SOCS) proteins are negative regulators of cytokine signaling. They act as feedback inhibitors to control the intensity and duration of cytokine signaling. SOCS proteins are induced by cytokine stimulation and can inhibit JAK-STAT signaling by binding to activated receptors, JAKs, or downstream signaling molecules, thus preventing further signaling. SOCS proteins help maintain immune homeostasis and prevent excessive immune activation. |
| Nuclear Factor of Activated T cells (NFAT) | Nuclear factor of activated T cells (NFAT) is a transcription factor involved in T cell cytokine signaling. NFAT is activated by calcium signaling pathways downstream of T cell receptor (TCR) activation. Once activated, NFAT translocates to the nucleus and regulates the expression of genes encoding cytokines, such as IL-2 and IFN-γ. NFAT is critical for T cell activation, cytokine production, and T cell effector functions. |
These molecules, along with many others, collectively contribute to the intricate network of T cell cytokine signaling. They regulate cytokine production, receptor signaling, and downstream cellular responses, ultimately shaping the immune response and influencing various immune-related processes. Understanding the roles and interactions of these molecules is crucial for deciphering the mechanisms of T cell cytokine signaling and developing targeted therapeutic interventions.
Regulation of T Cell Cytokine Signaling in Health and Disease
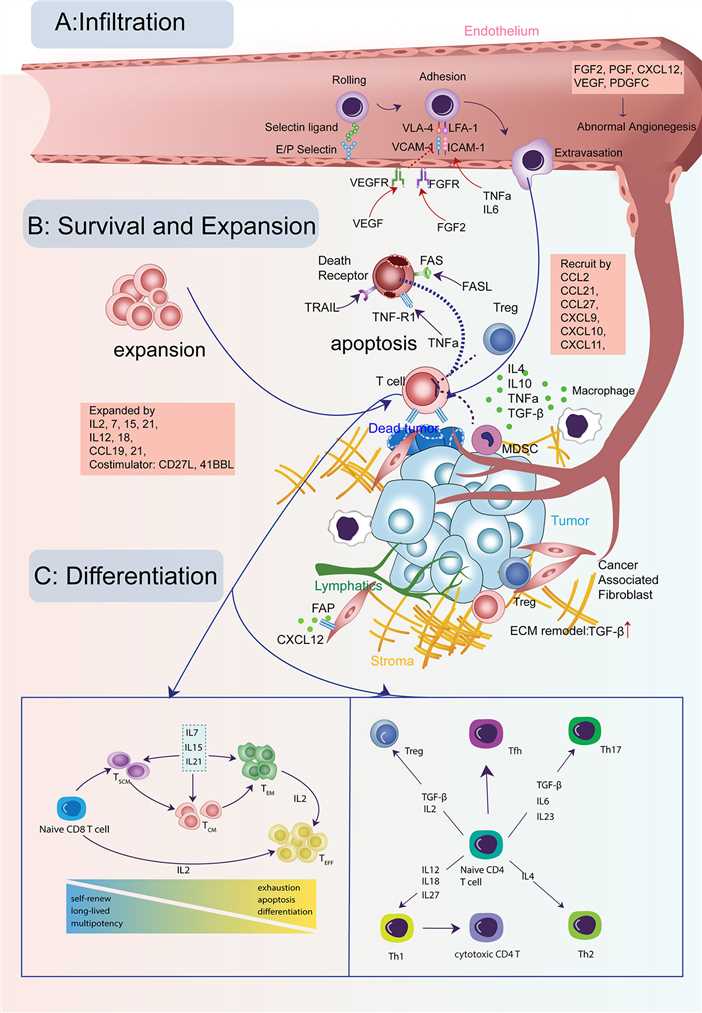 Fig.2 Cytokine signals in the life-span of T cells in tumors. (Zhang Y, et al., 2020)
Fig.2 Cytokine signals in the life-span of T cells in tumors. (Zhang Y, et al., 2020)The regulation of T cell cytokine signaling is of critical importance in maintaining immune homeostasis and preventing the development of immune-related disorders. Here are some key aspects of the regulation of T cell cytokine signaling in both health and disease:
Transcriptional Regulation
The production of cytokines by T cells is tightly regulated at the transcriptional level. Transcription factors, such as T-bet, GATA-3, RORγt, and Foxp3, play crucial roles in directing the differentiation and cytokine production of different T cell subsets. These transcription factors bind to specific gene regulatory regions and control the expression of cytokine genes. Dysregulation of these transcription factors can lead to altered cytokine profiles and contribute to the development of immune disorders.
Epigenetic Regulation
Epigenetic modifications, such as DNA methylation and histone modifications, also influence T cell cytokine signaling. Changes in the epigenetic landscape can affect the accessibility of cytokine gene loci, thereby modulating cytokine expression. Epigenetic regulators, including DNA methyltransferases, histone deacetylases, and chromatin remodeling complexes, help maintain the appropriate cytokine expression patterns in T cells. Disruptions in epigenetic regulation can contribute to aberrant cytokine production and immune dysregulation.
Signaling Pathways
T cell cytokine signaling is tightly regulated by various signaling pathways. The activation of T cell receptors (TCRs) by antigen recognition triggers intracellular signaling cascades, including the activation of protein kinases and transcription factors, which ultimately regulate cytokine production. Co-stimulatory molecules, such as CD28, and inhibitory receptors, such as CTLA-4 and PD-1, modulate TCR signaling and cytokine production. Perturbations in these signaling pathways can lead to altered cytokine responses and immune dysfunction.
Regulatory T Cells (Tregs)
Tregs are a specialized subset of T cells that play a crucial role in immune regulation. They express the transcription factor Foxp3 and produce anti-inflammatory cytokines, such as IL-10 and TGF-β. Tregs suppress the activation and cytokine production of other T cells, thereby maintaining immune tolerance and preventing excessive inflammation. Dysregulation of Treg function or reduced Treg numbers can result in uncontrolled immune responses and autoimmune diseases.
Environmental Factors and Microbiota
Environmental factors, such as diet, microbial exposure, and infections, can influence T cell cytokine signaling. For example, certain dietary components and metabolites derived from the gut microbiota can modulate T cell differentiation and cytokine production. Environmental factors can interact with genetic factors to shape the cytokine profiles of T cells and affect immune responses. Imbalances in the gut microbiota or alterations in environmental exposures can contribute to immune dysregulation and disease development.
Dysregulated T cell cytokine signaling is implicated in various immune-related disorders, including autoimmune diseases, allergic reactions, chronic inflammation, and cancer. Understanding the mechanisms underlying the regulation of T cell cytokine signaling in health and disease is crucial for developing targeted therapies aimed at restoring immune balance and treating immune-mediated disorders.
Case Study
Case 1: Tamiya T, Kashiwagi I, Takahashi R, Yasukawa H, Yoshimura A. Suppressors of cytokine signaling (SOCS) proteins and JAK/STAT pathways: regulation of T-cell inflammation by SOCS1 and SOCS3. Arterioscler Thromb Vasc Biol. 2011;31(5):980-985.
T-cell differentiation from naı̈ve cells into the various functional subtypes (eg, Th1, Th2, Th17, and Treg cells) primarily depends on the action of cytokines as indicated. SOCS1 and SOCS3 regulate the cytokine pathways indicated and thereby dictate CD4 T-cell differentiation. Note that SOCS3 inhibits Th1 by overexpression by suppressing IL-12-medaited STAT4 activation; however, SOCS3 deletion also inhibits Th1 by promoting production of IL-10 and TGF- . In Tregs, SOCS1 deletion promotes Treg expansion; however, these are functionally impaired. Thus, SOCS1 is negatively regulates Treg number but is necessary for the function of Tregs. The role of SOCS3 in Tregs has not been clarified. For Th17, ROR t induction by TGFhas been shown to be independent of Smad2/3/4.
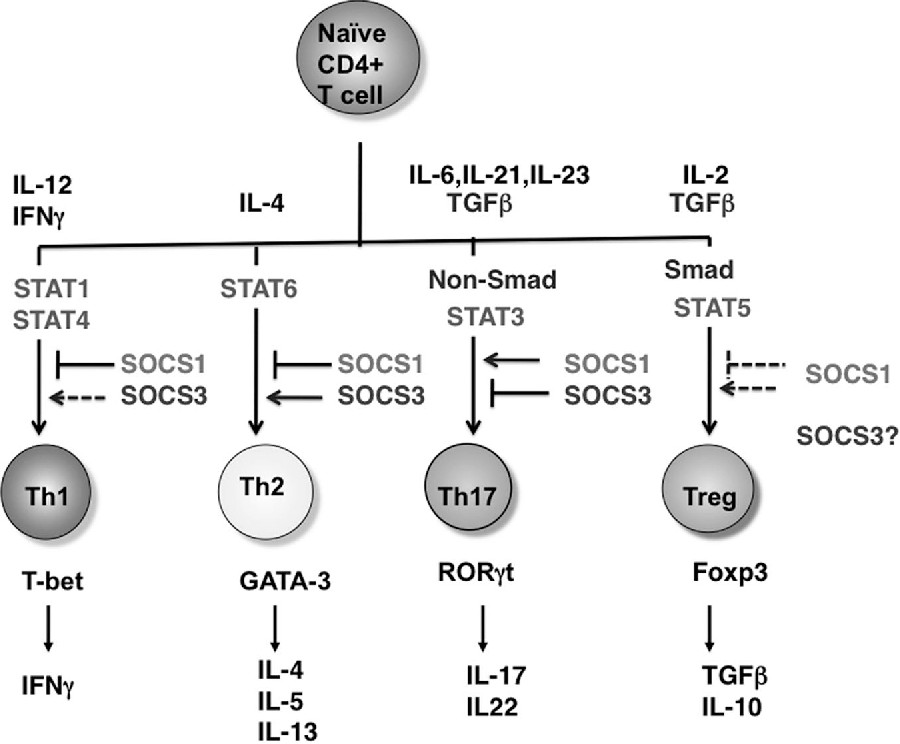 Fig.1 Role of STATs and SOCS proteins in Th-cell differentiation.
Fig.1 Role of STATs and SOCS proteins in Th-cell differentiation.Case 2: Banerjee A, Li D, Guo Y, et al. A reengineered common chain cytokine augments CD8+ T cell-dependent immunotherapy. JCI Insight. 2022;7(10):e158889.
Enhanced NFAT signaling mediated by OMCPmutIL-2 is the result of augmented TCR signal transduction. NFAT activation is mediated by TCR-initiated calcium flux. In the presence of TCR stimulation, OMCPmutIL-2 mediated robust signal transduction, as measured by upregulation of Nur77 and gene expression analysis of TCR-specific genes. No such signaling was evident in the absence of TCR ligation. Interestingly, TCR signal transduction was augmented by IL-2 and IL-15 as well, albeit much less than that evident in the presence of OMCPmutIL-2.
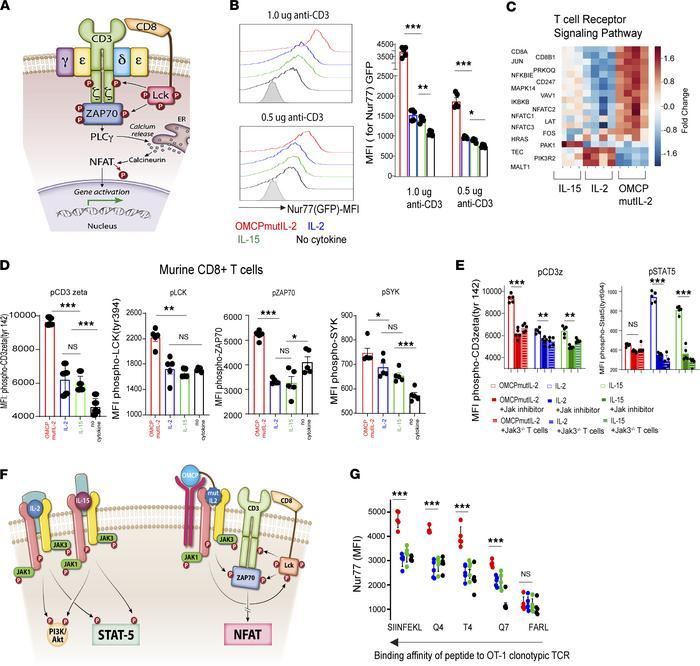 Fig.2 OMCPmutIL-2 promotes NFAT-mediated CD8+ T cell activation by augmenting TCR signal transduction.
Fig.2 OMCPmutIL-2 promotes NFAT-mediated CD8+ T cell activation by augmenting TCR signal transduction.References
- Dong C. Cytokine Regulation and Function in T Cells. Annu Rev Immunol. 2021;39:51-76.
- Zhang Y, Guan XY, Jiang P. Cytokine and Chemokine Signals of T-Cell Exclusion in Tumors. Front Immunol. 2020 Dec 14;11:594609.
- Wu J, Xie A, Chen W. Cytokine regulation of immune tolerance. Burns Trauma. 2014;2(1):11-17.

