Receptors in the Jak/STAT Pathway
Related Symbol Search List
- AGTR1
- CCR1
- CCR2
- CCR5
- CNTFR
- CSF1R
- CSF3R
- CXCR4
- Her2
- ERBB2
- ErbB4
- F2R
- FGF1
- FLT1
- FLT4
- Growth Hormone
- IFNGR1
- IL28A
- IGF1R
- IL10RA
- IL10RB
- IL11RA
- IL12RB1
- IL12RB2
- IL15
- IL15RA
- IL20RB
- IL21
- IL21R
- IL23R
- IL2RA
- IL2RB
- IL32
- IL4R
- IL5RA
- Il6ra
- IL6ST
- IL7R
- IL9R
- INSR
- Kdr
- KIT
- LEPR
- LIFR
- MERTK
- MPL
- TrkB
- OSMR
- PDGFRA
- PPP2CB
- Prlr
- PTAFR
- TIE2
Immunology Background
About Receptors in the Jak/STAT Pathway
The Jak/STAT (Janus kinase/signal transducer and activator of transcription) pathway is an intracellular signaling pathway that mediates the action of a large number of cytokines and growth factors involved in a variety of cellular processes including cell growth, differentiation, and immune responses. The pathway is initiated by the binding of cytokines or growth factors to specific receptors on the cell surface. These receptors activate Janus kinase (Jaks) to rephosphorylate and activate downstream transcription factors (i.e. signal transducer and activator of transcription (STAT) proteins).
Activators of the Jak/STAT signaling pathway include type I/II interferons, IL-6, IL-10, IL-12, the common gamma-chain, and common beta-chain cytokine families, as well as some growth factors that signal through homodimeric receptors, receptor tyrosine kinases, and G protein-coupled receptors. This signaling pathway regulates erythropoiesis, immune cell proliferation and differentiation, and inflammatory responses.
Understanding the receptors that activate the Jak/STAT pathway is critical to elucidating the signaling mechanisms and developing therapeutic strategies to target this pathway.
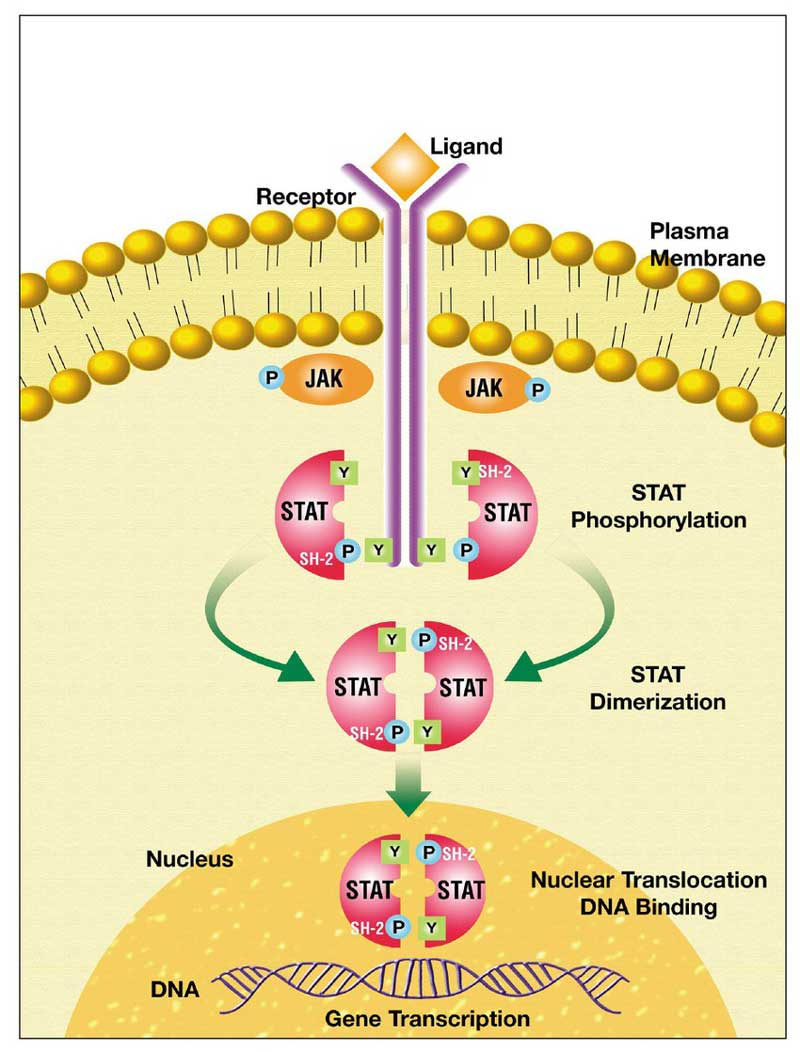 Fig.1 JAK-STAT signal transduction pathway. Ligand-induced receptor. (Benekli M, et al., 2003)
Fig.1 JAK-STAT signal transduction pathway. Ligand-induced receptor. (Benekli M, et al., 2003)
Mechanism of Action of Receptors in the Jak/STAT Pathway
Receptors in the Jak/STAT pathway employ specific mechanisms to initiate signaling. These receptors typically belong to the cytokine receptor superfamily or receptor tyrosine kinase family. Upon ligand binding, the receptors undergo conformational changes that lead to the activation of associated Jaks. Activated Jaks phosphorylate specific tyrosine residues on the cytoplasmic domain of the receptor, creating docking sites for STAT proteins. The phosphorylated STATs then bind to the receptors, allowing Jaks to further phosphorylate the STATs. This phosphorylation triggers their dimerization, nuclear translocation, and subsequent transcriptional activation of target genes.
Functions of Receptors in the Jak/STAT Pathway
Receptors in the Jak/STAT pathway play critical roles in a wide range of biological processes. Here are some examples of their functions:
- Immune Response
Receptors such as Interleukin receptors (ILRs) and Interferon receptors (IFNRs) activate the Jak/STAT pathway in immune cells, leading to the regulation of immune responses, including cytokine production, cell proliferation, and differentiation.
- Growth and Development
Certain receptors in the Jak/STAT pathway, such as growth hormone receptors (GHRs), are involved in regulating growth and development processes. They mediate the effects of growth factors and hormones on cell growth, proliferation, and differentiation.
- Hematopoiesis
Receptors like erythropoietin receptors (EPORs) and Thrombopoietin receptors (TPORs) activate the Jak/STAT pathway in hematopoietic cells, stimulating the production and maturation of red and white blood cells, as well as platelets.
- Inflammation
Receptors, including those for Interleukin-6 (IL-6) and Interleukin-23 (IL-23), contribute to the regulation of inflammatory responses. Activation of the Jak/STAT pathway by these receptors leads to the production of pro-inflammatory cytokines and the modulation of immune cell function.
Available Resources for Jak/STAT Pathway Positive Regulators
Understanding the receptors and their functions in the Jak/STAT pathway is critical to elucidating the complexity of cell signaling and developing targeted therapeutics. At Creative BioMart, we offer a comprehensive range of high-quality research tools and services to support studies related to the Jak/STAT pathway, including receptor proteins, ligands, inhibitors, and custom assay development. The receptors listed below have been shown to activate the Jak/STAT pathway. Click to view all related molecules/targets and research reagents. Don't hesitate to get in touch with us with any questions or requests.
Please feel free to explore our website for more information and contact our expert team for further assistance in your research endeavors.
Reference:
- Benekli M, Baer M R, Baumann H, et al.Signal transducer and activator of transcription proteins in leukemias.[J].Blood, 2003(8).

