Negative Regulators of the Jak/STAT Pathway
Related Symbol Search List
- JAK1
- AGTR1
- BLK
- BRCA1
- CAMK2B
- CaMKII
- CAMK2G
- CCR1
- CCR2
- CCR5
- CNTFR
- CSF1R
- CSF3R
- CXCR4
- Her2
- ERBB2
- ErbB4
- F2R
- FGF1
- FLT1
- FLT4
- FOS
- FYN
- Growth Hormone
- IFNGR1
- IL28A
- IGF1R
- IKBKE
- IL10RA
- IL10RB
- IL11RA
- IL12RB1
- IL12RB2
- IL15
- IL15RA
- IL20RB
- IL21
- IL21R
- IL23R
- IL2RA
- IL2RB
- IL32
- IL4R
- IL5RA
- Il6ra
- IL6ST
- IL7R
- IL9R
- INSR
- JUN
- Kdr
- KIT
- LEPR
- LIFR
- Lyn
- ERK2
- MAPK11
- MAPK14
- ERK1
- MAPK3
- MAPK8
- MERTK
- MPL
- TrkB
- OSMR
- PDGFRA
- PPP2CB
- PRKCD
- Prlr
- PTAFR
- RORC
- SH2B1
- Src
- STAT1
- STAT2
- STAT3
- STAT4
- STAT6
- STIP1
- TIE2
- YES1
Immunology Background
About Negative Regulators of the Jak/STAT Pathway
Cytokines induce a variety of biological responses by binding to specific cell surface receptors and activating cytoplasmic signaling pathways (e.g., the JAK/STAT pathway). Signaling through the Jak/STAT pathway is tightly controlled by several distinct mechanisms as dysregulation of Jak/STAT signaling is thought to underlie several disorders including rheumatologic and autoimmune disorders, transplant rejection, and several cancers. Key negative regulators of this pathway include Suppressors of Cytokine Signaling (SOCS), Protein Inhibitors of Activated STATs (PIASs), and protein tyrosine phosphatases (PTPs). The SOCS proteins are the primary regulators of the Jak/STAT pathway. They act as pseudo-Jak substrates to inhibit Jak/STAT signaling. They also block STAT activation and target multiple components of the Jak/STAT pathway for Ubiquitin-mediated proteasomal degradation. PIAS proteins interact with activated STAT dimers and inhibit STAT-mediated transcription. These regulatory proteins also modulate the DNA-binding properties of STATs by facilitating SUMOylation of the dimers. Jak/STAT signaling is also regulated by numerous constitutively expressed PTPs that dephosphorylate important tyrosine residues on multiple components of the pathway, thereby attenuating Jak/STAT signaling. Thus, the duration and intensity of the cellular response to cytokines appear to be determined by the net effect of several regulatory mechanisms.
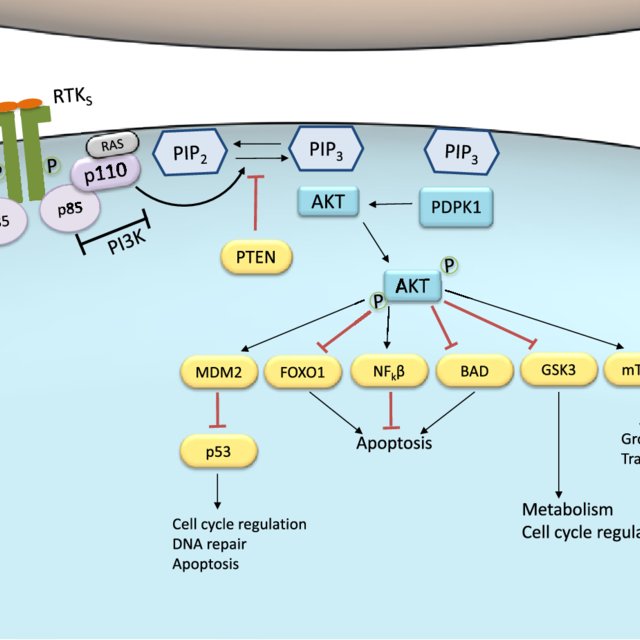 Fig.1 JAK-STAT pathway activation and its negative regulators. (Dantonio P M, et al., 2018)
Fig.1 JAK-STAT pathway activation and its negative regulators. (Dantonio P M, et al., 2018)
Mechanism of Negative Regulators of the Jak/STAT Pathway
Negative regulators employ diverse mechanisms to constrain the Jak/STAT pathway.
- Suppressors of Cytokine Signaling (SOCS)
SOCS proteins are one of the major negative regulators of the Jak/STAT pathway. They are induced by STAT activation and act as a negative feedback loop to inhibit further signaling. SOCS proteins, including SOCS1, SOCS2, SOCS3, and CIS, bind to activated Jaks and cytokine receptors, leading to their degradation or blocking the recruitment of downstream signaling molecules.
- Protein Inhibitors of Activated STATs (PIAS)
PIAS proteins are another class of negative regulators that exert their inhibitory effects by directly interacting with activated STATs. PIAS proteins, such as PIAS1, PIAS3, and PIASy, mediate the sumoylation of STATs, which prevents their DNA binding and transcriptional activity. Additionally, PIAS proteins can also recruit corepressors and histone deacetylases to inhibit STAT-mediated gene expression.
- Suppressor of Tumorigenicity (STATT)
STATT is a negative regulator that specifically targets STAT3. It competes with STAT3 for binding to cytokine receptors, preventing the activation of STAT3 signaling. Additionally, STATT can recruit protein phosphatases to dephosphorylate and inactivate STAT3, further attenuating its activity.
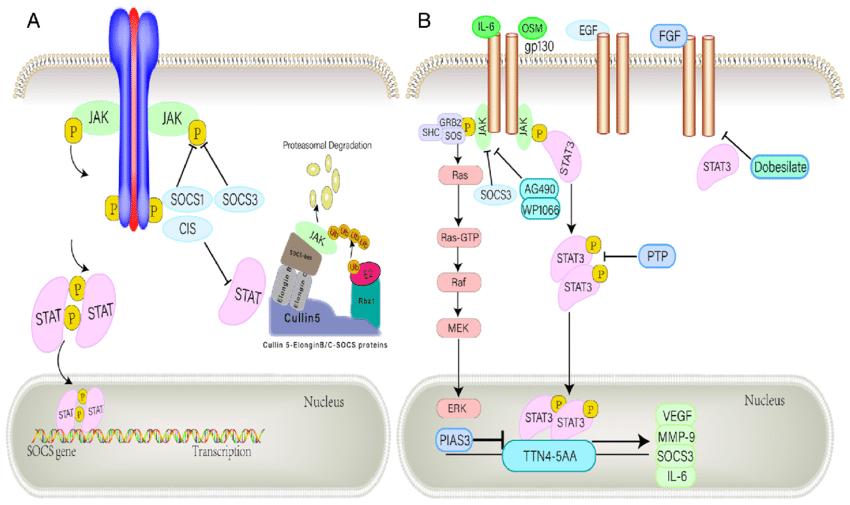 Fig.2 Negative regulation of the JAK/STAT pathway. (Dai L, et al., 2022)
Fig.2 Negative regulation of the JAK/STAT pathway. (Dai L, et al., 2022)
Functions of Negative Regulators of the Jak/STAT Pathway
- Immune Regulation
Negative regulators of the Jak/STAT pathway play a critical role in modulating immune responses. By attenuating the Jak/STAT signaling, they prevent excessive inflammation and immune cell activation. Dysregulation of these negative regulators can lead to autoimmune diseases, chronic inflammation, and immunodeficiency.
- Development and Differentiation
The Jak/STAT pathway is involved in cell differentiation and development. Negative regulators ensure proper control and timing of these processes. They fine-tune the signaling output, allowing for precise regulation of cell fate determination and tissue development.
- Tumor Suppression
Dysregulation of the Jak/STAT pathway is often associated with cancer development. Negative regulators, such as SOCS proteins, act as tumor suppressors by inhibiting aberrant Jak/STAT signaling. Their downregulation or inactivation can contribute to uncontrolled cell growth, metastasis, and resistance to therapy.
Available Resources for Negative Regulators of the Jak/STAT Pathway
Negative regulators of the Jak/STAT pathway are essential for maintaining proper cellular homeostasis and preventing pathological conditions. Understanding their mechanisms and functions provides valuable insights for therapeutic interventions aimed at modulating the Jak/STAT signaling pathway. Creative BioMart offers a wide range of products and services related to Jak/STAT pathway research, including recombinant proteins, antibodies, and assay kits, to facilitate the study of these negative regulators and their potential applications in various fields. Click to view all related molecules/targets and research reagents. Please get in touch with us with any questions or requests.
References:
- Dai L, Li Z, Liang W, et al. SOCS proteins and their roles in the development of glioblastoma[J]. Oncology Letters, 2022, 23(1): 1-13.
- Dantonio P M, Klein M O, Freire M R V B, et al. Exploring major signaling cascades in melanoma genesis: a rationale route for targetted skin cancer therapy[J]. Bioscience reports, 2018, 38(5): BSR20180511.
