Interleukin Subfamily
Jump to Section
Interleukins are a diverse group of cytokines that play critical roles in immune system communication, inflammation, and the regulation of cellular responses. Each IL has unique structural and functional properties, but they often work in concert to shape immune outcomes in health and disease.
The interleukin superfamily is generally classified into several subfamilies based on structural features, receptor usage, and functional similarities. Major subgroups include the IL-1 family (e.g., IL-1α, IL-1β, IL-18), known for pro-inflammatory activity; the common γ-chain (γc) family (e.g., IL-2, IL-4, IL-7, IL-9, IL-15, IL-21), which share a common receptor component and regulate lymphocyte development and homeostasis; the IL-6 family, which signal through gp130 and are involved in inflammation and hematopoiesis; and the IL-10 family, known for anti-inflammatory effects. By exploring IL-1 through IL-21, this article highlights both the individuality and interconnected nature of these powerful immune mediators.
Explore the interleukin (IL) family with Creative BioMart, which introduces members IL-1 through IL-21 in sequential order.
IL-1
IL-1 was originally termed a pyrogenic factor due to its role in inducing fever. It is a cytokine mainly produced by activated macrophages and exists in two forms: IL-1α and IL-1β. The interleukin-1 family (IL-1F) has an ancient evolutionary history. Based on amino acid sequence conservation, gene structure homology, and three-dimensional structure, 11 IL-1 family members have been identified—7 pro-inflammatory agonists (IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β, and IL-36γ) and 4 anti-inflammatory antagonists (IL-1Ra, IL-36Ra, IL-37, and IL-38). The biological activities of IL-1α and IL-1β are mediated by the IL-1 receptor type I (IL-1R1), which undergoes a conformational change upon ligand binding and recruits the IL-1 receptor accessory protein (IL-1RAcP). This receptor complex initiates intracellular signaling through the MyD88-dependent pathway, activating the NF-κB, p38 MAPK, JNK and ERK pathways. IL-1Ra acts as a natural inhibitor, competing for IL-1R1 binding without recruiting IL-1RAcP, thereby preventing downstream signaling. IL-1R2 acts as a decoy receptor, either membrane-bound or in a soluble form (sIL-1R2), sequestering IL-1 ligands and dampening the inflammatory response.
IL-1 is a key mediator of inflammation that regulates cellular immune activation. It plays a central role in both acute and chronic inflammation triggered by various stimuli (e.g. antigens, endotoxins, bacteria and viruses) and is implicated in the pathogenesis of diseases such as type 2 diabetes, rheumatoid arthritis and periodontitis. In addition to its immunological roles, IL-1 is involved in the regulation of the hematopoietic, nervous, and endocrine systems and contributes to antitumor immune responses. Monitoring IL-1 levels can provide valuable insights for diagnosis, treatment evaluation and prognosis in various inflammatory diseases.
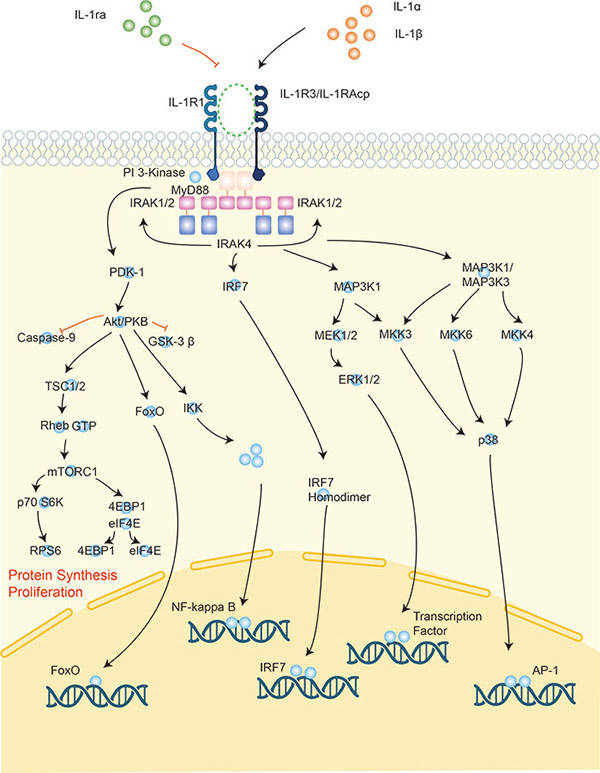
Figure 1. Signaling pathway of interleukin-1.
IL-2
Without stimulation from external signals, T cells cannot survive or proliferate long-term in vitro. The addition of interleukin-2 (IL-2) enables sustained proliferation of T cells, which is why IL-2 was originally called T cell growth factor (TCGF). However, resting (naive) T cells do not express high-affinity IL-2 receptors (IL-2R) and must first be activated by mitogens or antigens to induce IL-2R expression, making them responsive to IL-2 signaling. IL-2 also enhances its own signaling by inducing the upregulation of IL-2Rα (CD25) on target T cells. In vivo, IL-2 primarily acts on CD4+ T cells via autocrine signaling, since activated CD4+ T cells produce significant amounts of IL-2 themselves. IL-2R expression is transient, typically peaking 2–3 days post-activation and declining around day 6–10, after which T cells lose their sensitivity to IL-2. Therefore, for long-term culture of T cells in vitro, continuous stimulation with mitogens, antigens, or cytokines is required to maintain IL-2R expression and cell viability.
IL-3
IL-3 is an important early hematopoietic growth factor that plays a critical role in the regulation of hematopoiesis. It acts primarily on multipotent hematopoietic stem and progenitor cells, promoting their survival, proliferation and differentiation. IL-3 exerts its effects in the early stages of blood cell development and works synergistically with other growth factors—such as erythropoietin (EPO), granulocyte-macrophage colony-stimulating factor (GM-CSF), and thrombopoietin (TPO)—in later stages to support lineage-specific differentiation.
IL-3 supports the formation of multiple colony-forming units (CFUs), including:
- CFU-GM (granulocyte-macrophage progenitors)
- CFU-G (granulocyte progenitors)
- CFU-M (macrophage progenitors)
- CFU-Meg (megakaryocyte progenitors)
- CFU-Eo (eosinophil progenitors)
- CFU-Bas (basophil progenitors)
- CFU-E (erythroid progenitors)
Notably, IL-3 does not act on fully mature cells but plays a supportive role in vivo hematopoiesis, particularly in stress or emergency hematopoiesis, such as during infection or bone marrow injury.
IL-4
IL-4 is a pleiotropic cytokine primarily produced by activated Th2 cells, as well as mast cells and eosinophils. It plays a central role in the development and differentiation of T and B lymphocytes and is a key driver of humoral immune responses, particularly IgE class switching in B cells. IL-4 is not primarily known for activating cytotoxic T cells (CD8+ T cells) but rather for promoting Th2 cell differentiation and suppressing Th1-type immune responses. It exerts broad immunoregulatory effects on B cells, T cells, mast cells, macrophages, and hematopoietic cells, influencing processes such as proliferation, survival, and cytokine production.
IL-5
IL-5, also known as eosinophil differentiation factor and eosinophil colony-stimulating factor, is a pleiotropic cytokine produced primarily by Th2 cells (a subset of CD4+ T cells), as well as by mast cells and eosinophils. It plays a critical role in the production, activation and survival of eosinophils, particularly in the bone marrow where it promotes eosinophil differentiation. Transgenic mice overexpressing IL-5 exhibit significant eosinophilia, with increased numbers of eosinophils in the peripheral blood, spleen and bone marrow.
IL-5 enhances eosinophil functions such as degranulation, which is essential for pathogen defense, particularly against parasitic infections. It also promotes eosinophil activation, which contributes to tumor cell killing through mechanisms that may overlap with antibody-dependent cellular cytotoxicity (ADCC). In addition, IL-5 increases the production of reactive oxygen species, including superoxide anions, and prolongs the survival of eosinophils in peripheral blood. IL-5 is also involved in the mucosal immune response and plays a key role in conditions such as asthma and allergic diseases where eosinophilic inflammation is prevalent.
IL-6
IL-6 is primarily produced by monocytes, macrophages, fibroblasts, vascular endothelial cells, and T cells, especially Th2 cells. It acts on a variety of target cells, including macrophages, hepatocytes, activated B cells, plasma cells, and resting T cells, exhibiting a broad range of biological effects. Historically, IL-6 has been referred to by various names, including B cell stimulating factor-2 (BSF-2), 26kDa protein, B cell differentiation factor (BCDF), and hepatocyte stimulating factor (HSF).
IL-6 has diverse immunological roles, including antitumor effects where it enhances the cytolytic activity of natural killer (NK) cells and cytotoxic T cells. It also stimulates the production of glucocorticoids, enhances osteoclast activity, and promotes keratinocyte growth. IL-6 plays a key role in hematopoiesis, particularly in the bone marrow. It supports the survival and differentiation of various immune cells.
However, IL-6 alone cannot induce the secretion of other cytokines in target cells. At physiological concentrations, its autocrine effects on immune cells are relatively weak. Thus, IL-6 primarily functions to amplify the effects of other cytokines, rather than acting independently. This makes it a critical mediator in both acute-phase responses and chronic inflammatory diseases.
IL-7
IL-7 acts primarily on lymphocytes, including B cell progenitors, thymocytes, and mature T cells in both primary lymphoid organs (such as the bone marrow and thymus) and secondary lymphoid tissues (such as lymph nodes and spleen). IL-7 exerts growth-promoting activity on B cell progenitors, thymocytes, and mature T cells and plays a critical role in lymphocyte development, survival, and homeostasis by regulating the proliferation and differentiation of these different lymphocyte lineages.
In addition to its role in B cells and T cells, IL-7 also supports NK cell homeostasis, although it is not the primary cytokine for NK cell development. As a key cytokine supporting lymphopoiesis, IL-7 signaling is essential for the development and function of the adaptive immune system.
IL-8
IL-8 (also known as CXCL8) is a chemokine secreted primarily by Th1 cells, although it can also be produced by a variety of other cell types, including macrophages, endothelial cells, and fibroblasts. It plays a key role in chemotaxis, attracting neutrophils to sites of infection or injury and activating them to promote neutrophil degranulation, phagocytic activity, and the production of reactive oxygen species (ROS) that contribute to the respiratory burst.
IL-8 is a multifunctional factor with broad species specificity and a wide range of biological functions. Originally recognized for its ability to recruit neutrophils to inflammatory tissues, it also plays a role in acute inflammation by promoting acute-phase protein synthesis, inducing fever, and contributing to tissue damage. IL-8 is involved in the release of inflammatory mediators and fibroblast proliferation, which is critical in the context of chronic inflammation.
In addition to its role in neutrophil activation, IL-8 is involved in a variety of other inflammatory processes, including stimulation of angiogenesis (blood vessel formation), induction of mitosis in certain cell types, and regulation of the host immune response. It has been implicated in the pathogenesis of several inflammatory diseases, tumors and immune disorders.
IL-9
In humans, IL-9, also known as P40, is a cytokine secreted by T cells, particularly Th2 cells. In mice, IL-9 is also known as P40, mast cell growth-enhancing factor (MCGF), and T-cell growth factor III. While mouse IL-9 can act on both mouse and human cells, human IL-9 generally does not show significant activity on mouse cells, highlighting the species specificity of its effects.
IL-9 has bidirectional immunological functions; it can both promote the development of certain diseases and suppress the development of others. Current research suggests that IL-9 may act as a pathogenic factor in conditions such as hypersensitivity reactions and autoimmune diseases. On the other hand, in the context of parasitic infections and skin transplants, IL-9 plays a protective role, helping the body to eliminate pathogens and promoting the development of immune tolerance to transplanted tissues.
The immunoregulatory roles of IL-9 are complex, as it can either facilitate or inhibit the progression of various diseases. In addition, there is clear species specificity in IL-9 receptor activation, with IL-9 exerting different effects in humans and mice, reflecting the need for species-specific approaches in immunological research and therapeutic strategies.
IL-10
IL-10, also known as cytokine synthesis inhibitory factor (CSIF), is a pleiotropic cytokine that can exert both immunosuppressive and immunostimulatory effects, depending on the context and cell types involved. Primarily produced by monocytes/macrophages, IL-10 plays a critical role in regulating immune responses by limiting inflammation. As a key negative regulator, it inhibits the production of pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, and IL-8, as well as prostaglandin E2. In addition, IL-10 suppresses the proliferation of T cell subsets, including Th0, Th1, and Th2-like clones, thereby preventing excessive immune activation. Through these mechanisms, IL-10 helps maintain immune homeostasis and protects against pathologic inflammation.
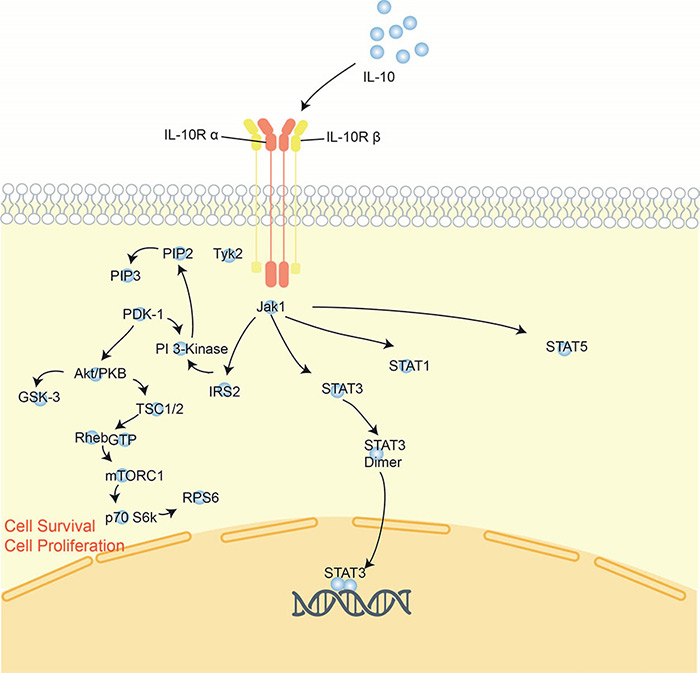
Figure 2. Signaling pathway of interleukin-10.
IL-11
IL-11 is a cytokine produced primarily by human bone marrow stromal cells (fibroblasts) and mesenchymal cells, with additional secretion by the human thyroid carcinoma cell line NTM-1. IL-11 plays a role in both primary and secondary immune responses by influencing antigen-specific antibody responses. In vitro, IL-11 is known to promote IL-6-dependent plasmacytoma cell proliferation and T-cell-dependent B-cell development. In addition, IL-11 can induce the differentiation of primitive cells into macrophages when cultured with erythropoietin (EPO). IL-11 exerts broad immunoregulatory effects by supporting the growth and differentiation of various immune cells, both directly and through interaction with other cytokines. It also plays an important role in hematopoiesis and in bridging the immune and hematopoietic systems, making it important in the coordination of immune responses and blood cell formation.
IL-12
IL-12 is produced by dendritic cells (DCs), macrophages, B lymphocytes and other antigen-presenting cells (APCs). IL-12 p70 is a critical cytokine in cell-mediated immunity, with its primary role being the induction of naive T helper cell differentiation into the Th1 subset. This cytokine also promotes the development, proliferation and functional activity of Th1 cells. Th1 cells are essential for both cellular and humoral immune responses, which are largely mediated by B lymphocytes.
Helper T cells can be divided into two subsets: Th1 and Th2. Th1 cells produce cytokines such asIL-2, IL-3, IFN-γ, lymphotoxin, and granulocyte macrophage-colony stimulating factor (GM-CSF), all of which are involved in cellular immunity. In contrast, Th2 cells produce IL-4, IL-5, IL-10 and IL-13, which primarily support B cell antibody production and play a key role in humoral immunity.
As a key inducer of Th1 polarization, IL-12 is central to bridging innate and adaptive immunity, particularly in response to intracellular pathogens and tumor cells. By driving the Th1 immune response, IL-12 helps protect against infection by intracellular pathogens and contributes to anti-tumor immunity.
IL-13
IL-13 is a cytokine secreted by various cell types, including CD4+ T cells, CD8+ T cells, mast cells, eosinophils, basophils, and natural killer (NK) cells. IL-13 has regulatory effects on monocyte morphology and expression of surface molecules. It promotes morphological changes in monocytes/macrophages and accelerates their fusion to form foreign body giant cells (FBGCs), which are involved in the response to chronic inflammation or foreign material. IL-13 increases the expression of CD23 (a low affinity IgE receptor), major histocompatibility complex II (MHC II), and mannose receptors on human monocytes. These receptors are involved in antigen presentation and phagocytosis, respectively.
In addition, IL-13 can inhibit gene transcription and protein synthesis of inflammatory cytokines such as IL-1, IL-6, and IL-8, which are typically activated by lipopolysaccharide (LPS) in monocytes/macrophages. By modulating these pathways, IL-13 plays a key role in the defense response, antigen presentation, and resolution of inflammation.
IL-15
IL-15 is a soluble cytokine that plays a critical role in the regulation of inflammatory and immune responses in the body. It acts as a chemoattractant for various immune cells, including T cells. IL-15 stimulates the proliferation of cytotoxic T lymphocyte (CTL) lines, phytohemagglutinin (PHA)-activated T cells, and tumor-infiltrating lymphocytes. It also acts synergistically with IL-2 to promote immune responses. Notably, IL-15 is chemotactic for T cells, and its chemotactic effect is stronger than that of IL-8 and macrophage inflammatory protein-α (MIP-α).
As a multifunctional cytokine, IL-15 regulates both innate and adaptive immunity by activating and recruiting different immune cell populations involved in inflammatory responses. Its key functions include enhancing T cell proliferation, supporting the survival of memory T cells, and participating in the coordination of immune responses against infections and tumors.
IL-16
IL-16 is primarily produced by activated CD8+ T cells, but can also be produced by other immune cells such as eosinophils and dendritic cells. Its receptor is CD4, which is expressed on the surface of CD4+ T cells and some other immune cells. IL-16 acts as a chemoattractant, recruiting CD4+ T cells, monocytes and eosinophils to sites of immune activation. It also induces the expression of the IL-2 receptor (IL-2R) and MHC class II molecules (HLA-DR) on T cells and monocytes.
As an immunoregulatory chemokine, IL-16 plays a critical role in the recruitment and activation of CD4+ T cells and antigen-presenting cells (APCs) through interaction with the CD4 receptor. This engagement triggers various immune responses and contributes to both innate and adaptive immunity. The sequential effects of IL-16 on different immune cell populations help to coordinate the immune response, including antigen presentation and T cell activation.
IL-17
IL-17A is a potent pro-inflammatory cytokine that plays a critical role in amplifying both innate and adaptive immune responses. It promotes the local production of chemoattractants such as IL-8, MCP-1 (monocyte chemoattractant protein-1), and Gro-α (growth-regulated α-protein), which facilitate the recruitment of monocytes and neutrophils to sites of inflammation. In addition, IL-17A can stimulate the production of hematopoietic growth factors such as granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF), thereby promoting the proliferation of myeloid cell lineages, including neutrophils, in the bone marrow.
IL-17A also upregulates the expression of intercellular adhesion molecules (ICAMs) on endothelial cells and other target cells, thereby enhancing the recruitment and activation of immune cells, particularly T cells, to the site of inflammation. By triggering multiple downstream signaling pathways, IL-17A plays a key role in infection defense and modulation of tissue inflammation.
While its pro-inflammatory properties are essential for pathogen clearance, excessive or prolonged IL-17A signaling can contribute to the development of immunopathology, such as chronic inflammation and autoimmune diseases. Thus, while IL-17A is essential for immune defense, its dysregulation can exacerbate tissue damage and disease progression.
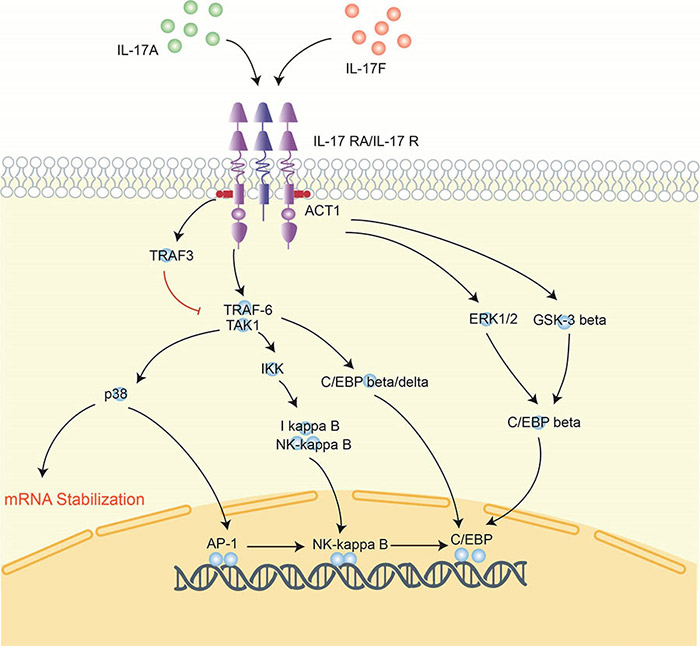
Figure 3. Signaling pathway of interleukin-17.
IL-18
IL-18 is a multifunctional cytokine that plays a critical role in immune and inflammatory responses, with effects that are not species specific. Originally discovered for its role in neutrophil activation, IL-18 recruits these cells to sites of inflammation where it induces degranulation and the production of reactive oxygen species (ROS), leading to a respiratory burst. In addition to neutrophil activation, IL-18 promotes the release of inflammatory mediators and acute phase proteins that contribute to fever and acute inflammation.
IL-18 also plays a role in the pathogenesis of chronic inflammation by stimulating fibroblast proliferation and the release of additional inflammatory cytokines. In addition to its effects on neutrophils, IL-18 can act on a variety of other immune and non-immune cells to promote further inflammatory processes, enhance angiogenesis (blood vessel formation), induce mitosis, and regulate immune cell functions.
IL-18 has been implicated in a wide range of diseases, including various inflammatory disorders, autoimmune diseases and certain types of tumors. Its involvement in both innate and adaptive immune responses underscores its importance in host defense, but also in the pathogenesis of chronic inflammatory and immune-mediated diseases.
IL-19
IL-19 is a member of the IL-10 cytokine family, which also includes IL-10, IL-20, IL-22, and IL-24. It is expressed primarily in human monocytes, B lymphocytes, T lymphocytes, and other inflammatory cells. IL-19 exerts its biological functions primarily through binding to the IL-20R1/IL-20R2 receptor complex, although its precise role in immune regulation remains somewhat controversial.
IL-19 has been implicated in both pro-inflammatory and anti-inflammatory responses, although its overall effects depend on the cellular context. Some studies suggest that IL-19 may exert anti-inflammatory effects similar to IL-10 by inducing a phenotypic switch in T helper cells (e.g., from Th1 to Th2), which is generally associated with an anti-inflammatory response. Conversely, other studies have shown that IL-19 can enhance the production of pro-inflammatory cytokines such as IL-6 and TNF-α, particularly in monocytes, suggesting a potential pro-inflammatory role.
Although IL-20R1 expression has not been detected on most immune cells, many studies indicate that IL-19 has significant effects on immune cells, particularly within the Th2-associated immune system. This suggests that while IL-19 signals through the IL-10 receptor complex, its role in immune responses may involve a complex interplay of both pro- and anti-inflammatory effects, likely dependent on the specific immune context.
IL-20
IL-20 is a recently discovered cytokine that belongs to the IL-10 cytokine family, which includes IL-10, IL-19, IL-20, IL-22 and IL-24. IL-20 plays a critical role in regulating the physiological functions of the skin, primarily by activating the STAT3 signaling pathway in keratinocytes. This activation induces the upregulation of various cytokines and chemokines, which in turn modulate early inflammatory responses in the skin.
Through its effects on keratinocytes, IL-20 is involved in maintaining skin homeostasis and may also contribute to the initiation of cutaneous inflammation. It plays an important role in several skin diseases, including psoriasis, where overexpression of IL-20 is associated with pathological inflammation. In addition, the regulation of IL-20 signaling networks provides valuable insights into the immunological mechanisms that control skin health and disease.
IL-21
IL-21 is a cytokine that belongs to the common γ-chain cytokine receptor family, which includes other cytokines such as IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 itself. Like other members of this family, IL-21 is a type I cytokine that forms a four-helical alpha-band structure. It signals through a receptor complex consisting of the IL-21 receptor (IL-21R) and the common γ chain (IL-2Rγ).
IL-21 is produced primarily by CD4+ T cells, natural killer T (NKT) cells, and a subset of CD8+ T cells. It has broad effects on a variety of immune cell types. In CD4+ T cells, IL-21 is essential for the differentiation of Th17 cells and the generation of T follicular helper (Tfh) cells, which are critical for supporting B cell differentiation and antibody production in germinal centers. IL-21 also regulates B cell functions by promoting their proliferation, survival, and immunoglobulin production, as well as facilitating isotype switching, although its effects on B cells may be context-dependent.
In addition, IL-21 enhances the cytotoxic functions of CD8+ T cells, natural killer cells, and NKT cells, thereby boosting the body's innate immune response. In summary, IL-21 integrates both innate and adaptive immune responses by modulating various immune cell subsets, making it a key cytokine for immune regulation.
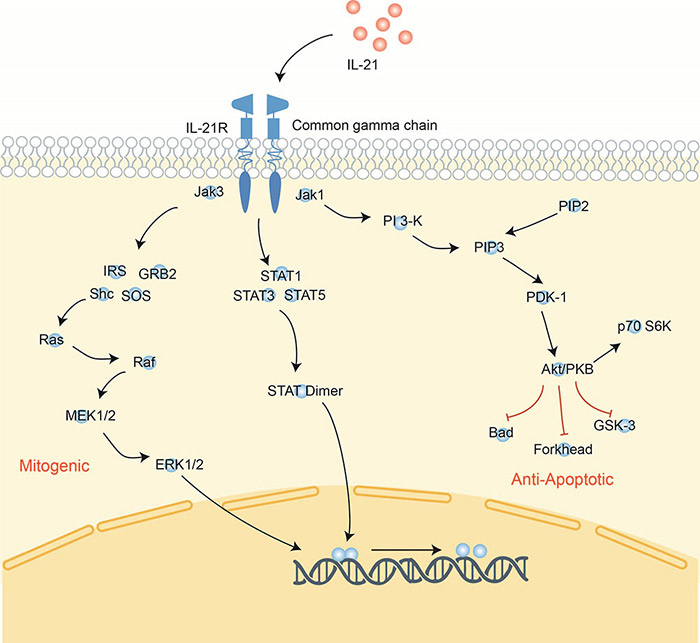
Figure 4. Signaling pathway of interleukin-21.
Related Products
- IL
- IL Pathway
- IL Family related Drugs
- IL-1 Family
- IL-1 Family Ligands
- IL-1 Family Receptors
- IL-1 Family Signaling Molecules
- IL-6 Family
- IL-6 Family Ligands
- IL-6 Family Receptors
- IL-6 Signaling Related Molecules
- IL-10 Family
- IL-10 Family Ligands
- IL-10 Family Receptors
- IL-10 Signaling Related Molecules
- IL-12 Family
- IL-12 Family Ligands
- IL-12 Family Receptors
- IL-12 Signaling Related Molecules
- IL-17 Family
- IL-17 Family Receptors
- IL-17 Family Ligands
- IL-17 Signaling Related Molecules
References
- Hall BK. Chapter 15 - cells to make and cells to break. In: Hall BK, ed. Bones and Cartilage. Academic Press; 2005:197-213. doi:10.1016/B978-012319060-4/50017
- Justiz Vaillant AA, Qurie A. Interleukin. 2022 Aug 22. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 29763015.
- Shouse AN, LaPorte KM, Malek TR. Interleukin-2 signaling in the regulation of T cell biology in autoimmunity and cancer. Immunity. 2024;57(3):414-428. doi:10.1016/j.immuni.2024.02.001
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

